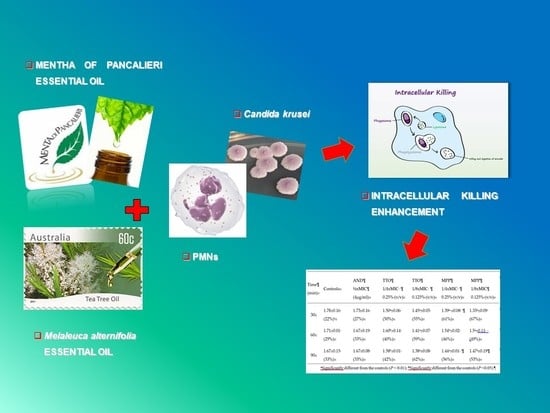
Enhanced Killing of Candida krusei by Polymorphonuclear Leucocytes in the Presence of Subinhibitory Concentrations of Melaleuca alternifolia and “Mentha of Pancalieri” Essential Oils
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Yeasts
4.2. Essential Oils
4.3. Reference Antifungal Agent
4.4. In Vitro Antifungal Susceptibility Assays
4.5. Human Polymorphonuclear Leucocytes (PMNs)
4.6. Influence of Antimicrobial Agents on Intracellular Killing by PMNs
4.7. PMNs Viability Evaluation in the Presence of EOs
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rodrigues, M.E.; Silva, S.; Azeredo, J.; Henriques, M. Novel strategies to fight Candida infection. Crit. Rev. Microbiol. 2016, 42, 594–606. [Google Scholar] [CrossRef] [PubMed]
- Swamy, M.K.; Akhtar, M.S.; Sinniah, U.R. Antimicrobial properties of plant essential oils against human pathogens and their mode of action: An updated review. Evid. Based Complement. Altern. Med. 2016, 2016, 3012462. [Google Scholar] [CrossRef] [PubMed]
- Anibal, P.C.; De Cássia Orlandi Sardi, J.; Peixoto, I.T.; de Carvalho Moraes, J.J.; Höfling, J.F. Conventional and alternative antifungal therapies to oral candidiasis. Braz. J. Microbiol. 2010, 41, 824–831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharifi-Rad, J.; Sureda, A.; Tenore, G.C.; Daglia, M.; Sharifi-Rad, M.; Valussi, M.; Tundis, R.; Sharifi-Rad, M.; Loizzo, M.R.; et al. Biological activities of essential oils: From plant chemoecology to traditional healing systems. Molecules 2017, 22, 70. [Google Scholar] [CrossRef] [PubMed]
- Tullio, V.; Cuffini, A.M.; Banche, G.; Mandras, N.; Allizond, V.; Roana, J.; Giacchino, F.; Bonello, F.; Ungheri, D.; Carlone, N.A. Role of fosfomycin tromethamine in modulating non-specific defence mechanisms in chronic uremic patients towards ESBL-producing Escherichia coli. Int. J. Immunopathol. Pharm. 2008, 21, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Tullio, V.; Mandras, N.; Scalas, D.; Allizond, V.; Banche, G.; Roana, J.; Greco, D.; Castagno, F.; Cuffini, A.M.; Carlone, N.A. Synergy of caspofungin with human polymorphonuclear granulocytes for killing Candida albicans. Antimicrob. Agents Chemother. 2010, 54, 3964–3966. [Google Scholar] [CrossRef] [PubMed]
- Banche, G.; Allizond, V.; Mandras, N.; Tullio, V.; Cuffini, A.M. Host immune modulation by antimicrobial drugs: Current knowledge and implications for antimicrobial chemotherapy. Curr. Opin. Pharm. 2014, 18, 159–166. [Google Scholar] [CrossRef]
- Wojtunik-Kulesza, K.; Kasprzak, K.; Oniszczuk, T.; Oniszczuk, A. Natural monoterpenes: Much more than only a scent. Chem. Biodivers. 2019. [Google Scholar] [CrossRef]
- Pazyar, N.; Yaghoobi, R.; Bagherani, N.; Kazerouni, A. A review of applications of tea tree oil in dermatology. Int. J. Derm. 2013, 52, 784–790. [Google Scholar] [CrossRef]
- Satchell, A.C.; Saurajen, A.; Bell, C.; Barnetson, R.S. Treatment of dandruff with 5% tea tree oil shampoo. J. Am. Acad. Derm. 2002, 47, 852–855. [Google Scholar] [CrossRef] [Green Version]
- Carson, C.F.; Hammer, K.A.; Riley, T.V. Melaleuca alternifolia (Tea Tree) Oil: A review of antimicrobial and other medicinal properties. Clin. Microbiol. Rev. 2006, 19, 50–62. [Google Scholar] [CrossRef] [PubMed]
- Di Vito, M.; Mattarelli, P.; Modesto, M.; Girolamo, A.; Ballardini, M.; Tamburro, A.; Meledandri, M.; Mondello, F. In vitro activity of tea tree oil vaginal suppositories against Candida spp. and probiotic vaginal microbiota. Phytother. Res. 2015, 29, 1628–1633. [Google Scholar] [CrossRef] [PubMed]
- Di Campli, E.; Di Bartolomeo, S.; Delli Pizzi, P.; Di Giulio, M.; Grande, R.; Nostro, A.; Cellini, L. Activity of tea tree oil and nerolidol alone or in combination against Pediculus capitis (head lice) and its eggs. Parasitol. Res. 2012, 111, 1985–1992. [Google Scholar] [CrossRef] [PubMed]
- Mertas, A.; Garbusińska, A.; Szliszka, E.; Jureczko, A.; Kowalska, M.; Król, W. The influence of tea tree oil (Melaleuca alternifolia) on fluconazole activity against fluconazole-resistant Candida albicans strains. Biomed. Res. Int. 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- McKay, D.L.; Blumberg, J.B. A review of the bioactivity and potential health benefits of peppermint tea (Mentha piperita L.). Phytother. Res. 2006, 20, 619–633. [Google Scholar] [CrossRef] [PubMed]
- Alammar, N.; Wang, L.; Saberi, B.; Nanavati, J.; Holtmann, G.; Shinohara, R.T.; Mullin, G.E. The impact of peppermint oil on the irritable bowel syndrome: A meta-analysis of the pooled clinical data. BMC Complement. Altern. Med. 2019, 19, 21. [Google Scholar] [CrossRef] [PubMed]
- Elsaie, L.T.; El Mohsen, A.M.; Ibrahim, I.M.; Mohey-Eddin, M.H.; Elsaie, M.L. Effectiveness of topical peppermint oil on symptomatic treatment of chronic pruritus. Clin. Cosmet. Investig. Derm. 2016, 9, 333–338. [Google Scholar] [CrossRef]
- Tardugno, R.; Pellati, F.; Iseppi, R.; Bondi, M.; Bruzzesi, G.; Benvenuti, S. Phytochemical composition and in vitro screening of the antimicrobial activity of essential oils on oral pathogenic bacteria. Nat. Prod. Res. 2018, 32, 544–551. [Google Scholar] [CrossRef]
- Rajkowska, K.; Kunicka-Styczyńska, A.; Maroszyńska, M. Selected essential oils as antifungal agents against antibiotic-resistant Candida spp.: In vitro study on clinical and food-borne isolates. Microb. Drug Resist. 2017, 23, 18–24. [Google Scholar] [CrossRef]
- Carretto, C.F.P.; Almeida, R.B.A.; Furlan, M.R.; Jorge, A.O.C.; Junqueira, J.C. Antimicrobial activity of Mentha piperita L. against Candida spp. Braz. Dent. Sci. 2010, 13, 4–9. [Google Scholar] [CrossRef]
- Tullio, V.; Roana, J.; Scalas, D.; Mandras, N. Evaluation of the antifungal activity of Mentha x piperita (Lamiaceae) of Pancalieri (Turin, Italy) essential oil and its synergistic interaction with azoles. Molecules 2019, 24, 3148. [Google Scholar] [CrossRef]
- Turner, S.A.; Butler, G. The Candida pathogenic species complex. Cold Spring Harb. Perspect. Med. 2014, 4, a019778. [Google Scholar] [CrossRef] [PubMed]
- Pfaller, M.A.; Diekema, D.J.; Gibbs, D.L.; Newell, V.A.; Nagy, E.; Dobiasova, S.; Rinaldi, M.; Barton, R.; Veselov, A.; Global Antifungal Surveillance Group. Candida krusei, a multidrug-resistant opportunistic fungal pathogen: Geographic and temporal trends from the ARTEMIS DISK Antifungal Surveillance Program, 2001 to 2005. J. Clin. Microbiol. 2008, 46, 515–521. [Google Scholar] [CrossRef] [PubMed]
- Córdoba, S.; Vivot, W.; Szusz, W.; Albo, G. Antifungal activity of essential oils against Candida species isolated from clinical samples. Mycopathologia 2019, 29. [Google Scholar] [CrossRef] [PubMed]
- Mandras, N.; Nostro, A.; Roana, J.; Scalas, D.; Banche, G.; Ghisetti, V.; Del Re, S.; Fucale, G.; Cuffini, A.M.; Tullio, V. Liquid and vapour-phase antifungal activities of essential oils against Candida albicans and non-albicans Candida. BMC Complement. Altern Med. 2016, 16, 330. [Google Scholar] [CrossRef]
- Tullio, V.; Cuffini, A.M.; Giacchino, F.; Mandras, N.; Roana, J.; Comune, L.; Merlino, C.; Carlone, N.A. Combined action of fluconazole and PMNs from uremic patients in clearing intracellular Candida albicans. J. Chemother. 2003, 15, 301–303. [Google Scholar] [CrossRef]
- Tullio, V.; Mandras, N.; Allizond, V.; Nostro, A.; Roana, J.; Merlino, C.; Banche, G.; Scalas, D.; Cuffini, A.M. Positive interaction of thyme (red) essential oil with human PMNs in eradicating intracellular Candida albicans. Planta Med. 2012, 78, 1633–1635. [Google Scholar] [CrossRef]
- Kothari, V. Working with natural products (extracts): Certain useful suggestions to avoid trouble. AASCIT Commun. 2014, 1, 37–39. [Google Scholar]
- Stevens, D.A.; Espiritu, M.; Parmar, R. Paradoxical effect of caspofungin: Reduced activity against Candida albicans at high concentrations. Antimicrob. Agents Chemother. 2004, 48, 3407–3411. [Google Scholar] [CrossRef]
- Cosentino, M.; Luini, A.; Bombelli, R.; Corasaniti, M.T.; Bagetta, G.; Marino, F. The essential oil of bergamot stimulates reactive oxygen species production in human polymorphonuclear leukocytes. Phytother Res. 2014, 28, 1232–1239. [Google Scholar] [CrossRef]
- Li, J.; Dong, J.; Qiu, J.Z.; Wang, J.F.; Luo, M.J.; Li, H.E.; Leng, B.F.; Ren, W.Z.; Deng, X.M. Peppermint oil decreases the production of virulence-associated exoproteins by Staphylococcus aureus. Molecules 2011, 16, 1642–1654. [Google Scholar] [CrossRef] [PubMed]
- Samber, N.; Khan, A.; Varma, A.; Manzoor, N. Synergistic anti-candidal activity and mode of action of Mentha piperita essential oil and its major components. Pharm. Biol. 2015, 53, 1496–1504. [Google Scholar] [CrossRef]
- Cox, S.D.; Mann, C.M.; Markham, J.L.; Bell, H.C.; Gustafson, J.E.; Warmington, J.R.; Wyllie, S.G. The mode of antimicrobial action of the essential oil of Melaleuca alternifolia (tea tree oil). J. Appl. Microbiol. 2000, 88, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Schepetkin, I.A.; Kushnarenko, S.V.; Özek, G.; Kirpotina, L.N.; Sinharoy, P.; Utegenova, G.A.; Abidkulova, K.T.; Özek, T.; Başer, K.H.; Kovrizhina, A.R.; et al. Modulation of human neutrophil responses by the essential oils from Ferula akitschkensis and their constituents. J. Agric. Food Chem. 2016, 64, 7156–7170. [Google Scholar] [CrossRef] [PubMed]
- Özek, G.; Schepetkin, I.A.; Utegenova, G.A.; Kirpotina, L.N.; Andrei, S.R.; Özek, T.; Başer, K.H.C.; Abidkulova, K.T.; Kushnarenko, S.V.; Khlebnikov, A.I.; et al. Chemical composition and phagocyte immunomodulatory activity of Ferula iliensis essential oils. J. Leukoc. Biol. 2017, 101, 1361–1371. [Google Scholar] [CrossRef] [PubMed]
- Sieniawska, E.; Michel, P.; Mroczek, T.; Granica, S.; Skalicka-Woźniak, K. Nigella damascena L. essential oil and its main constituents, damascenine and β-elemene modulate inflammatory response of human neutrophils ex vivo. Food Chem. Toxicol. 2019, 125, 161–169. [Google Scholar] [CrossRef]
- Basholli-Salihu, M.; Schuster, R.; Hajdari, A.; Mulla, D.; Viernstein, H.; Mustafa, B.; Mueller, M. Phytochemical composition, anti-inflammatory activity and cytotoxic effects of essential oils from three Pinus spp. Pharm. Biol. 2017, 55, 1553–1560. [Google Scholar] [CrossRef]
- Sun, Z.; Wang, H.; Wang, J.; Zhou, L.; Yang, P. Chemical composition and anti-inflammatory, cytotoxic and antioxidant activities of essential oil from leaves of Mentha piperita grown in China. PLoS ONE 2014, 9, e114767. [Google Scholar] [CrossRef]
- Scalas, D.; Mandras, N.; Roana, J.; Tardugno, R.; Cuffini, A.M.; Ghisetti, V.; Benvenuti, S.; Tullio, V. Use of Pinus sylvestris L. (Pinaceae), Origanum vulgare L. (Lamiaceae), and Thymus vulgaris L. (Lamiaceae) essential oils and their main components to enhance itraconazole activity against azole susceptible/not-susceptible Cryptococcus neoformans strains. BMC Complement. Altern. Med. 2018, 18, 143. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts-Third Edition: Approved Standard M27-A3; CLSI: Wayne, PA, USA, 2008; Volume 28, pp. 6–12. [Google Scholar]
- Clinical and Laboratory Standards Institute. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts; Fourth Informational Supplement. CLSI document M27-S4; CLSI: Wayne, PA, USA, 2012; Volume 27, pp. 6–12. [Google Scholar]
- Mandras, N.; Tullio, V.; Furneri, P.M.; Roana, J.; Allizond, V.; Scalas, D.; Petronio Petronio, G.; Fuochi, V.; Banche, G.; Cuffini, A.M. Key roles of human polymorphonuclear cells and ciprofloxacin in Lactobacillus species infection control. Antimicrob. Agents Chemother. 2015, 60, 1638–1641. [Google Scholar] [CrossRef] [PubMed]
- Cuffini, A.M.; Tullio, V.; Giacchino, F.; Mandras, N.; Scalas, D.; Belardi, P.; Merlino, C.; Carlone, N.A. Impact of co-amoxiclav on polymorphonuclear granulocytes from chronic hemodialysis patients. Am. J. Kidney Dis. 2001, 37, 1253–1259. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds TTO, “Mentha of Pancalieri” EO, anidulafungin and Candida krusei clinical strain are available from the authors. |

| Drug | 103 cfu/mL | 106 cfu/mL |
|---|---|---|
| Tea tree oil (TTO) | ||
| MIC | 0.5% v/v | 1% v/v |
| MFC | 0.5% v/v | 1% v/v |
| Mentha of Pancalieri EO | ||
| MIC | 0.25% v/v | 1% v/v |
| MFC | 0.5% v/v | 1% v/v |
| Anidulafungin (AND) | ||
| MIC | 1 μg/mL | 8 μg/mL |
| MFC | 16 μg/mL | >16 μg/mL |
| Survival Index ± SEM | ||||||
|---|---|---|---|---|---|---|
| Time (min) | Controls | AND 1/2 × MIC (4 μg/mL) | TTO 1/4 × MIC 0.25% (v/v) | TTO 1/8 × MIC 0.125% (v/v) | MPP 1/4 × MIC 0.25% (v/v) | MPP 1/8 × MIC 0.125% (v/v) |
| 30 | 1.78 ± 0.16 (22%)c | 1.73 ± 0.16 (27%) | 1.50 a ± 0.06 (50%) | 1.45 a ± 0.05 (55%) | 1.39 a ± 0.08 (61%) | 1.33 a ± 0.09 (67%) |
| 60 | 1.71 ± 0.01 (29%) | 1.67 ± 0.19 (33%) | 1.60 b ± 0.14 (40%) | 1.41 a ± 0.07 (59%) | 1.54 b ± 0.02 (46%) | 1.5 a ± 0.11 (49%) |
| 90 | 1.67 ± 0.15 (33%) | 1.67 ± 0.08 (33%) | 1.58 b ± 0.01 (42%) | 1.38 a ± 0.08 (62%) | 1.44 a ± 0.01 (56%) | 1.47 a ± 0.19(53%) |
| Survival Index ± SEM | ||||||
|---|---|---|---|---|---|---|
| Time (min) | Controls | AND 1/2 × MIC (4 μg/mL) | TTO 1/4 × MIC 0.25% (v/v) | TTO 1/8 × MIC 0.125% (v/v) | MPP 1/4 × MIC 0.25% (v/v) | MPP 1/8 × MIC 0.125% (v/v) |
| 30 | 1.78 ± 0.16 (22%)c | 1.83 ± 0.06 (17%) | 1.58 b ± 0.15 (42%) | 1.55 a ± 0.01 (45%) | 1.54 a ± 0.15 (46%) | 1.50 a ± 0.01 (50%) |
| 60 | 1.71 ± 0.01 (29%) | 1.82 ± 0.06 (18%) | 1.61 ± 0.14 (39%) | 1.57 a ± 0.06 (43%) | 1.56 b ± 0.14 (44%) | 1.53 a ± 0.06(47%) |
| 90 | 1.67 ± 0.15 (33%) | 1.87 ± 0.03 (13%) | 1.64 ± 0.04 (36%) | 1.61 a ± 0.01 (39%) | 1.43 a ± 0.04 (57%) | 1.40 a ± 0.01(60%) |
| Survival Index ± SEM | ||||||
|---|---|---|---|---|---|---|
| Time (min) | Controls | AND 1/2 × MIC (4 μg/mL) | TTO 1/4 × MIC 0.25% (v/v) | TTO 1/8 × MIC 0.125% (v/v) | MPP 1/4 × MIC 0.25% (v/v) | MPP 1/8 × MIC 0.125% (v/v) |
| 30 | 1.78 ± 0.16 (22%)c | 1.52 b ± 0.13 (48%) | 1.64 ± 0.05 (14%) | 1.83 ± 0.06 (17%) | 1.56 b ± 0.11 (44%) | 1.36 a ± 0.03 (64%) |
| 60 | 1.71 ± 0.01 (29%) | 1.55 b ± 0.15 (45%) | 1.88 ± 0.09 (12%) | 1.78 ± 0.02 (22%) | 1.70 ± 0.11 (30%) | 1.46 a ± 0.14 (54%) |
| 90 | 1.67 ± 0.15 (33%) | 1.40 a ± 0.08 (60%) | 1.88 ± 0.05 (12%) | 1.76 ± 0.11 (24%) | 1.83 ± 0.02 (27%) | 1.39 a ± 0.13(61%) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tullio, V.; Roana, J.; Scalas, D.; Mandras, N. Enhanced Killing of Candida krusei by Polymorphonuclear Leucocytes in the Presence of Subinhibitory Concentrations of Melaleuca alternifolia and “Mentha of Pancalieri” Essential Oils. Molecules 2019, 24, 3824. https://doi.org/10.3390/molecules24213824
Tullio V, Roana J, Scalas D, Mandras N. Enhanced Killing of Candida krusei by Polymorphonuclear Leucocytes in the Presence of Subinhibitory Concentrations of Melaleuca alternifolia and “Mentha of Pancalieri” Essential Oils. Molecules. 2019; 24(21):3824. https://doi.org/10.3390/molecules24213824
Chicago/Turabian StyleTullio, Vivian, Janira Roana, Daniela Scalas, and Narcisa Mandras. 2019. "Enhanced Killing of Candida krusei by Polymorphonuclear Leucocytes in the Presence of Subinhibitory Concentrations of Melaleuca alternifolia and “Mentha of Pancalieri” Essential Oils" Molecules 24, no. 21: 3824. https://doi.org/10.3390/molecules24213824