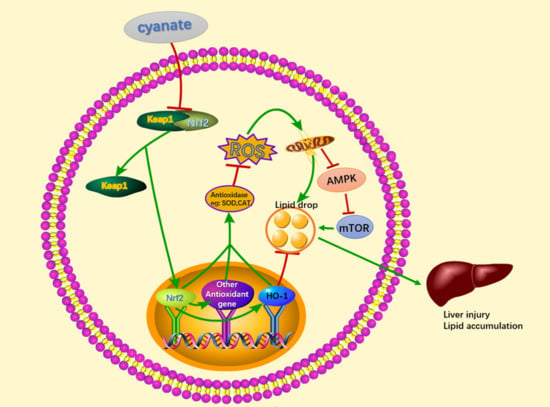
Cyanate Induces Oxidative Stress Injury and Abnormal Lipid Metabolism in Liver through Nrf2/HO-1
Abstract
:1. Introduction
2. Results
2.1. Cyanate Decreases Body Weight and Causes Dyslipidemia
2.2. Cyanate Reduced the Antioxidant Capacity of Liver
2.3. Cyanate Causes Liver Injury and Lipid Accumulation
2.4. Cyanate Inhabits the Nrf2 Pathway in Liver
2.5. Cyanate Decreases ROS Levels Caused Lipid Deposition in HL-7702 Cells
2.6. TBHQ Alleviates Oxidative Stress Caused by Cyanate in HL-7702 Cells
2.7. TBHQ Rescues the Inhibition Activity of the Nrf2 Pathway and the Activation of mTOR Pathway Caused by Cyanate
3. Discussion
4. Materials and Methods
4.1. Reagents and Antibodies
4.2. Cell Culture and Culture Condition
4.3. Cell Viability Assay
4.4. Reactive Oxygen Species Assay Kits
4.5. Immunoblot Assay
4.6. Immunofluorescence Staining
4.7. Detection of Oxidation-Associated Biological Indicators
4.8. Biochemical Analyses
4.9. Histological Analysis of Liver Steatosis
4.10. Liver Hematoxylin and Eosin Staining
4.11. Fibrosis Histology
4.12. Animal Models in C57BL/6 Mice
4.13. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| CKD | Chronic kidney disease |
| ESRD | End-stage renal disease |
| CVD | Cardiovascular disease |
| ROS | Reactive oxygen species |
| GFR | Glomerular filtration rate |
| ALT | Alanine aminotransferase |
| AST | Aspartate aminotransferase |
| ALP, | Alkaline phosphatase |
| LDH | Lactate dehydrogenase |
| CHE | Cholinesterase and |
| TC | Total cholesterol |
| TG | Triglycerides |
| LDL | Low-density lipoprotein-cholesterol |
| HDL | High-density lipoprotein-cholesterol |
| SOD | Superoxide dismutase |
| CAT | Catalase |
| MDA | Malondialdehyde |
| NO | Nitric oxide |
| Nrf2 | NF-E2-related factor 2 |
| Keap1 | Kelch-like ECH-associated protein 1 |
| HO-1 | Heme oxygenase-1 |
| AMPK | Adenosine 5‘-monophosphate (AMP)-activated protein kinase |
| mTOR | Mammalian target of rapamycin |
| DMEM | Dulbecco’s Modified Eagle Medium |
| DCFH-DA | Dichlorodihydrofluorescein diacetate |
| FBS | Fetal bovine serum |
References
- Webster, A.C.; Nagler, E.V.; Morton, R.L.; Masson, P. Chronic Kidney Disease. Lancet 2017, 389, 1238–1252. [Google Scholar] [CrossRef]
- Erratum: Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Update Work Group. KDIGO 2017 Clinical Practice Guideline Update for the Diagnosis, Evaluation, Prevention, and Treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int. Suppl. 2017, 7, 1–59. [CrossRef]
- Kotanko, P.; Rosales, L.; Ronco, C. Preface—Advances in CKD 2018. Blood Purif. 2018, 45, 151–153. [Google Scholar]
- Bello, A.K.; Levin, A.; Manns, B.J.; Feehally, J.; Drueke, T.; Faruque, L.; Hemmelgarn, B.R.; Kernahan, C.; Mann, J.; Klarenbach, S.; et al. Effective CKD care in European countries: Challenges and opportunities for health policy. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 2015, 65, 15–25. [Google Scholar] [CrossRef]
- Rutledge, J.C.; Ng, K.F.; Aung, H.H.; Wilson, D.W. Role of triglyceride-rich lipoproteins in diabetic nephropathy. Nat. Rev. Nephrol. 2010, 6, 361–370. [Google Scholar] [CrossRef]
- Oda, E.; Kawai, R. Low-density lipoprotein (LDL) cholesterol is cross-sectionally associated with preclinical chronic kidney disease (CKD) in Japanese men. Intern Med. 2010, 49, 713–719. [Google Scholar] [CrossRef]
- Weiner, D.E.; Tabatabai, S.; Tighiouart, H.; Elsayed, E.; Bansal, N.; Griffith, J.; Salem, D.N.; Levey, A.S.; Sarnak, M.J. Cardiovascular outcomes and all-cause mortality: Exploring the interaction between CKD and cardiovascular disease. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 2006, 48, 392–401. [Google Scholar] [CrossRef]
- Ballew, S.H.; Matsushita, K. Cardiovascular Risk Prediction in CKD. Semin. Nephrol. 2018, 38, 208–216. [Google Scholar] [CrossRef]
- Schiffrin, E.L.; Lipman, M.L.; Mann, J.F. Chronic kidney disease: Effects on the cardiovascular system. Circulation 2007, 116, 85–97. [Google Scholar] [CrossRef]
- Lin, T.H.; Chuang, S.Y.; Chu, C.Y.; Lee, W.H.; Hsu, P.C.; Su, H.M.; Li, Y.H.; Fang, C.C.; Wang, K.Y.; Tseng, W.K.; et al. The impact of chronic kidney disease on lipid management and goal attainment in patients with atherosclerosis diseases in Taiwan. Int. J. Med. Sci. 2014, 11, 381–388. [Google Scholar] [CrossRef]
- Bajaj, A.; Xie, D.; Cedillo-Couvert, E.; Charleston, J.; Chen, J.; Deo, R.; Feldman, H.I.; Go, A.S.; He, J.; Horwitz, E.; et al. Lipids, Apolipoproteins, and Risk of Atherosclerotic Cardiovascular Disease in Persons With CKD. Am. J. Kidney Dis. 2019, 73, 827–836. [Google Scholar] [CrossRef] [Green Version]
- Batista, M.C.; Welty, F.K.; Diffenderfer, M.R.; Sarnak, M.J.; Schaefer, E.J.; Lamon-Fava, S.; Asztalos, B.F.; Dolnikowski, G.G.; Brousseau, M.E.; Marsh, J.B. Apolipoprotein A-I, B-100, and B-48 metabolism in subjects with chronic kidney disease, obesity, and the metabolic syndrome. Metab. Clin. Exp. 2004, 53, 1255–1261. [Google Scholar] [CrossRef]
- Chade, A.R.; Rodriguez-Porcel, M.; Grande, J.P.; Zhu, X.; Sica, V.; Napoli, C.; Sawamura, T.; Textor, S.C.; Lerman, A.; Lerman, L.O. Mechanisms of renal structural alterations in combined hypercholesterolemia and renal artery stenosis. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 1295–1301. [Google Scholar] [CrossRef]
- Nilsson, L.; Lundquist, P.; Kagedal, B.; Larsson, R. Plasma cyanate concentrations in chronic renal failure. Clin. Chem. 1996, 42, 482–483. [Google Scholar]
- Hasuike, Y.; Nakanishi, T.; Moriguchi, R.; Otaki, Y.; Nanami, M.; Hama, Y.; Naka, M.; Miyagawa, K.; Izumi, M.; Takamitsu, Y. Accumulation of cyanide and thiocyanate in haemodialysis patients. Nephrol. Dial. Transplant. Off. Publ. Eur. Dial. Transpl. Assoc. Eur. Ren. Assoc. 2004, 19, 1474–1479. [Google Scholar] [CrossRef]
- Wang, Z.; Nicholls, S.J.; Rodriguez, E.R.; Kummu, O.; Hörkkö, S.; Barnard, J.; Reynolds, W.F.; Topol, E.J.; DiDonato, J.A.; Hazen, S.L. Protein carbamylation links inflammation, smoking, uremia and atherogenesis. Nat. Med. 2007, 13, 1176–1184. [Google Scholar] [CrossRef]
- Kalim, S.; Karumanchi, S.A.; Thadhani, R.I.; Berg, A.H. Protein carbamylation in kidney disease: Pathogenesis and clinical implications. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 2014, 64, 793–803. [Google Scholar] [CrossRef]
- Verbrugge, F.H.; Tang, W.H.; Hazen, S.L. Protein carbamylation and cardiovascular disease. Kidney Int. 2015, 88, 474–478. [Google Scholar] [CrossRef] [Green Version]
- Holy, E.W.; Akhmedov, A.; Speer, T.; Camici, G.G.; Zewinger, S.; Bonetti, N.; Beer, J.H.; Lüscher, T.F.; Tanner, F.C. Carbamylated Low-Density Lipoproteins Induce a Prothrombotic State Via LOX-1, Impact on Arterial Thrombus Formation In Vivo. J. Am. Coll. Cardiol. 2016, 68, 1664–1676. [Google Scholar] [CrossRef]
- Holy, E.W.; Akhmedov, A.; Speer, T.; Camici, G.G.; Zewinger, S.; Bonetti, N.; Beer, J.H.; Lüscher, T.F.; Tanner, F.C. Urea-induced ROS generation causes insulin resistance in mice with chronic renal failure. J. Clin. Investig. 2010, 120, 203–213. [Google Scholar]
- Himmelfarb, J.; Hakim, R.M. Oxidative stress in uremia. Curr. Opin. Nephrol. Hypertens. 2003, 12, 593–598. [Google Scholar] [CrossRef]
- Vaziri, N.D. Roles of oxidative stress and antioxidant therapy in chronic kidney disease and hypertension. Curr. Opin. Nephrol. Hypertens. 2004, 13, 93–99. [Google Scholar] [CrossRef]
- Pruchniak, M.P.; Arazna, M.; Demkow, U. Biochemistry of Oxidative Stress. Adv. Exp. Med. Biol. 2016, 878, 9–19. [Google Scholar]
- Bast, A.; Goris, R.J. Oxidative stress. Biochemistry and human disease. Pharm. Weekbl. Sci. 1989, 11, 199–206. [Google Scholar] [CrossRef]
- Zhou, T.; Prather, E.R.; Garrison, D.E.; Zuo, L. Interplay between ROS and Antioxidants during Ischemia-Reperfusion Injuries in Cardiac and Skeletal Muscle. Int. J. Mol. Sci. 2018, 19, 417. [Google Scholar] [CrossRef]
- San Miguel, S.M.; Opperman, L.A.; Allen, E.P.; Zielinski, J.; Svoboda, K.K. Bioactive polyphenol antioxidants protect oral fibroblasts from ROS-inducing agents. Arch. Oral Biol. 2012, 57, 1657–1667. [Google Scholar] [CrossRef]
- Poljsak, B.; Suput, D.; Milisav, I. Achieving the balance between ROS and antioxidants: When to use the synthetic antioxidants. Oxid. Med. Cell. Longev. 2013, 2013, 956792. [Google Scholar] [CrossRef]
- Gutteridge, J.M.; Halliwell, B. Antioxidants: Molecules, medicines, and myths. Biochem. Biophys. Res. Commun. 2010, 393, 561–564. [Google Scholar] [CrossRef]
- Jun, M.; Venkataraman, V.; Razavian, M.; Cooper, B.; Zoungas, S.; Ninomiya, T.; Webster, A.C.; Perkovic, V. Antioxidants for chronic kidney disease. Cochrane Database Syst. Rev. 2012, 10, CD008176. [Google Scholar] [CrossRef]
- Fan, J.L.; Cai, H.B.; Tan, W.S. Effect of regulating intracellular ROS with antioxidants on the ex vivo expansion of cord blood CD34+ cells. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi 2008, 24, 767–770. [Google Scholar]
- Chen, Q.; Peng, H.; Dong, L.; Chen, L.; Ma, X.; Peng, Y.; Dai, S.; Liu, Q. Activation of the NRF2-ARE signalling pathway by the Lentinula edodes polysaccharose LNT alleviates ROS-mediated cisplatin nephrotoxicity. Int. Immunopharmacol. 2016, 36, 1–8. [Google Scholar] [CrossRef]
- Dodson, M.; de la Vega, M.R.; Cholanians, A.B.; Schmidlin, C.J.; Chapman, E.; Zhang, D.D. Modulating NRF2 in Disease: Timing Is Everything. Annu. Rev. Pharmacol. Toxicol. 2019, 59, 555–575. [Google Scholar] [CrossRef]
- Periyasamy, P.; Shinohara, T. Age-related cataracts: Role of unfolded protein response, Ca mobilization, epigenetic DNA modifications, and loss of Nrf2/Keap1 dependent cytoprotection. Prog. Retin. Eye Res. 2017, 60, 1–19. [Google Scholar] [CrossRef]
- Mildenberger, J.; Johansson, I.; Sergin, I.; Kjøbli, E.; Damås, J.K.; Razani, B.; Flo, T.H.; Bjørkøy, G. N-3 PUFAs induce inflammatory tolerance by formation of KEAP1-containing SQSTM1/p62-bodies and activation of NFE2L2. Autophagy 2017, 13, 1664–1678. [Google Scholar] [CrossRef] [Green Version]
- Tsakiri, E.N.; Gumeni, S.; Vougas, K.; Pendin, D.; Papassideri, I.; Daga, A.; Gorgoulis, V.; Juhász, G.; Scorrano, L.; Trougakos, I.P. Proteasome dysfunction induces excessive proteome instability and loss of mitostasis that can be mitigated by enhancing mitochondrial fusion or autophagy. Autophagy 2019. [Google Scholar] [CrossRef]
- Xu, D.; Li, X.; Shao, F.; Lv, G.; Lv, H.; Lee, J.H.; Qian, X.; Wang, Z.; Xia, Y.; Du, L.; et al. The protein kinase activity of fructokinase A specifies the antioxidant responses of tumor cells by phosphorylating p62. Sci. Adv. 2019, 5, eaav4570. [Google Scholar] [CrossRef] [Green Version]
- Shanmugam, G.; Challa, A.K.; Litovsky, S.H.; Devarajan, A.; Wang, D.; Jones, D.P.; Darley-Usmar, V.M.; Rajasekaran, N.S. Enhanced Keap1-Nrf2 signaling protects the myocardium from isoproterenol-induced pathological remodeling in mice. Redox Biol. 2019. [Google Scholar] [CrossRef]
- Prasad, K.N. Simultaneous Activation of Nrf2 and Elevation of Dietary and Endogenous Antioxidant Chemicals for Cancer Prevention in Humans. J. Am. Coll. Nutr. 2016, 35, 175–184. [Google Scholar] [CrossRef]
- Liang, M.; Wang, Z.; Li, H.; Cai, L.; Pan, J.; He, H.; Wu, Q.; Tang, Y.; Ma, J.; Yang, L. l-Arginine induces antioxidant response to prevent oxidative stress via stimulation of glutathione synthesis and activation of Nrf2 pathway. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2018, 115, 315–328. [Google Scholar] [CrossRef]
- Bello, A.K.; Levin, A.; Tonelli, M.; Okpechi, I.G.; Feehally, J.; Harris, D.; Jindal, K.; Salako, B.L.; Rateb, A.; Osman, M.A.; et al. Assessment of Global Kidney Health Care Status. JAMA 2017, 317, 1864–1881. [Google Scholar] [CrossRef]
- Liu, M.; Grigoryev, D.N.; Crow, M.T.; Haas, M.; Yamamoto, M.; Reddy, S.P.; Rabb, H. Transcription factor Nrf2 is protective during ischemic and nephrotoxic acute kidney injury in mice. Kidney Int. 2009, 76, 277–285. [Google Scholar] [CrossRef] [Green Version]
- Vaziri, N.D.; Navab, M.; Fogelman, A.M. HDL metabolism and activity in chronic kidney disease. Nat. Rev. Nephrol. 2010, 6, 287–296. [Google Scholar] [CrossRef]
- Gisterå, A.; Hansson, G.K. The immunology of atherosclerosis. Nat. Rev. Nephrol. 2017, 13, 368–380. [Google Scholar] [CrossRef]
- Kuwahara, M.; Bannai, K.; Segawa, H.; Miyamoto, K.; Yamato, H. Cardiac remodeling associated with protein increase and lipid accumulation in early-stage chronic kidney disease in rats. Biochim. Biophys. Acta 2014, 1842, 1433–1443. [Google Scholar] [CrossRef] [Green Version]
- Agrawal, S.; Zaritsky, J.J.; Fornoni, A.; Smoyer, W.E. Dyslipidaemia in nephrotic syndrome: Mechanisms and treatment. Nat. Rev. Nephrol. 2018, 14, 57–70. [Google Scholar] [CrossRef]
- Khatiwada, S.; Rajendra, K.C.; Gautam, S.; Lamsal, M.; Baral, N. Thyroid dysfunction and dyslipidemia in chronic kidney disease patients. BMC Endocr. Disord. 2015, 15, 65. [Google Scholar] [CrossRef]
- Chauhan, V.; Vaid, M. Dyslipidemia in chronic kidney disease: Managing a high-risk combination. Postgrad. Med. 2009, 121, 54–61. [Google Scholar] [CrossRef]
- Shoji, T.; Nishizawa, Y. Plasma lipoprotein abnormalities in hemodialysis patients—Clinical implications and therapeutic guidelines. Ther. Apher. Dial. Off. Peer-Rev. J. Int. Soc. Apher. Jpn. Soc. Apher. Jpn. Soc. Dial. Ther. 2006, 10, 305–315. [Google Scholar] [CrossRef]
- Ritz, E.; Wanner, C. Lipid changes and statins in chronic renal insufficiency. J. Am. Soc. Nephrol. JASN 2006, 17, S226–S230. [Google Scholar] [CrossRef]
- Kennedy, D.J.; Chen, Y.; Huang, W.; Viterna, J.; Liu, J.; Westfall, K.; Tian, J.; Bartlett, D.J.; Tang, W.H.; Xie, Z.; et al. CD36 and Na/K-ATPase-α1 form a proinflammatory signaling loop in kidney. Hypertension 2013, 61, 216–224. [Google Scholar] [CrossRef]
- Gai, Z.; Wang, T.; Visentin, M.; Kullak-Ublick, G.A.; Fu, X.; Wang, Z. Lipid Accumulation and Chronic Kidney Disease. Nutrients 2019, 11, 722. [Google Scholar] [CrossRef]
- Reiss, A.B.; Voloshyna, I.; De Leon, J.; Miyawaki, N.; Mattana, J. Cholesterol Metabolism in CKD. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 2015, 66, 1071–1082. [Google Scholar] [CrossRef] [Green Version]
- Kang, S.S.; Mun, K.C.; Seo, J.H.; Choe, M.; Ha, E. Cyanate improves insulin sensitivity and hepatic steatosis in normal and high fat-fed mice: Anorexic and antioxidative effects. Chem. Biol. Interact. 2018, 279, 121–128. [Google Scholar] [CrossRef]
- Alfaradhi, M.Z.; Fernandez-Twinn, D.S.; Martin-Gronert, M.S.; Musial, B.; Fowden, A.; Ozanne, S.E. Oxidative stress and altered lipid homeostasis in the programming of offspring fatty liver by maternal obesity. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2014, 307, R26–R34. [Google Scholar] [CrossRef] [Green Version]
- Sokołowska, M.; Niedzielska, E.; Iciek, M.; Bilska, A.; Lorenc-Koci, E.; Włodek, L. The effect of the uremic toxin cyanate (CNO⁻) on anaerobic cysteine metabolism and oxidative processes in the rat liver: A protective effect of lipoate. Toxicol. Mech. Methods 2011, 21, 473–478. [Google Scholar] [CrossRef]
- Del Vecchio, L.; Locatelli, F.; Carini, M. What we know about oxidative stress in patients with chronic kidney disease on dialysis—Clinical effects, potential treatment, and prevention. Semin. Dial. 2011, 24, 56–64. [Google Scholar] [CrossRef]
- Dounousi, E.; Papavasiliou, E.; Makedou, A.; Ioannou, K.; Katopodis, K.P.; Tselepis, A.; Siamopoulos, K.C.; Tsakiris, D. Oxidative stress is progressively enhanced with advancing stages of CKD. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 2006, 48, 752–760. [Google Scholar] [CrossRef]
- Oberg, B.P.; McMenamin, E.; Lucas, F.L.; McMonagle, E.; Morrow, J.; Ikizler, T.A.; Himmelfarb, J. Increased prevalence of oxidant stress and inflammation in patients with moderate to severe chronic kidney disease. Kidney Int. 2004, 65, 1009–1016. [Google Scholar] [CrossRef] [Green Version]
- Ruiz, S.; Pergola, P.E.; Zager, R.A.; Vaziri, N.D. Targeting the transcription factor Nrf2 to ameliorate oxidative stress and inflammation in chronic kidney disease. Kidney Int. 2013, 83, 1029–1041. [Google Scholar] [CrossRef] [Green Version]
- Tepel, M.; van der Giet, M.; Statz, M.; Jankowski, J.; Zidek, W. The antioxidant acetylcysteine reduces cardiovascular events in patients with end-stage renal failure: A randomized, controlled trial. Circulation 2003, 107, 992–995. [Google Scholar] [CrossRef]
- Galvan, D.L.; Badal, S.S.; Long, J.; Chang, B.H.; Schumacker, P.T.; Overbeek, P.A.; Danesh, F.R. Real-time in vivo mitochondrial redox assessment confirms enhanced mitochondrial reactive oxygen species in diabetic nephropathy. Kidney Int. 2017, 92, 1282–1287. [Google Scholar] [CrossRef]
- Kirkman, D.L.; Muth, B.J.; Ramick, M.G.; Townsend, R.R.; Edwards, D.G. Role of mitochondria-derived reactive oxygen species in microvascular dysfunction in chronic kidney disease. Am. J. Physiol. Ren. Physiol. 2018, 314, F423–F429. [Google Scholar] [CrossRef]
- Scheuer, H.; Gwinner, W.; Hohbach, J.; Gröne, E.F.; Brandes, R.P.; Malle, E.; Olbricht, C.J.; Walli, A.K.; Gröne, H.J. Oxidant stress in hyperlipidemia-induced renal damage. Am. J. Physiol. Ren. Physiol. 2000, 278, F63–F74. [Google Scholar] [CrossRef]
- Yamamoto, M.; Kensler, T.W.; Motohashi, H. The KEAP1-NRF2 System: A Thiol-Based Sensor-Effector Apparatus for Maintaining Redox Homeostasis. Physiol. Rev. 2018, 98, 1169–1203. [Google Scholar] [CrossRef]
- Xue, M.; Qian, Q.; Adaikalakoteswari, A.; Rabbani, N.; Babaei-Jadidi, R.; Thornalley, P.J. Activation of NF-E2-related factor-2 reverses biochemical dysfunction of endothelial cells induced by hyperglycemia linked to vascular disease. Diabetes 2008, 57, 2809–2817. [Google Scholar] [CrossRef]
- Cuadrado, A.; Rojo, A.I.; Wells, G.; Hayes, J.D.; Cousin, S.P.; Rumsey, W.L.; Attucks, O.C.; Franklin, S.; Levonen, A.L.; Kensler, T.W.; et al. Therapeutic targeting of the NRF2 and KEAP1 partnership in chronic diseases. Nat. Rev. Drug Discov. 2019, 18, 295–317. [Google Scholar] [CrossRef] [Green Version]
- Bollong, M.J.; Lee, G.; Coukos, J.S.; Yun, H.; Zambaldo, C.; Chang, J.W.; Chin, E.N.; Ahmad, I.; Chatterjee, A.K.; Lairson, L.; et al. A metabolite-derived protein modification integrates glycolysis with KEAP1-NRF2 signalling. Nature 2018, 562, 600–604. [Google Scholar] [CrossRef]
- Li, Y.; Shi, J.; Sun, X.; Li, Y.; Duan, Y.; Yao, H. Theaflavic acid from black tea protects PC12 cells against ROS-mediated mitochondrial apoptosis induced by OGD/R via activating Nrf2/ARE signaling pathway. J. Nat. Med. 2019. [Google Scholar] [CrossRef]
- Xiao, X.; Song, D.; Cheng, Y.; Hu, Y.; Wang, F.; Lu, Z.; Wang, Y. Biogenic nanoselenium particles activate Nrf2-ARE pathway by phosphorylating p38, ERK1/2, and AKT on IPEC-J2 cells. J. Cell. Physiol. 2019, 234, 11227–11234. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, Y.; Huang, X.; Xie, Y.; Qu, Y.; Long, H.; Gu, N.; Jiang, W. Z-Ligustilide protects vascular endothelial cells from oxidative stress and rescues high fat diet-induced atherosclerosis by activating multiple NRF2 downstream genes. Atherosclerosis 2019, 284, 110–120. [Google Scholar] [CrossRef]
- Yuki, H.; Kamai, T.; Murakami, S.; Higashi, S.; Narimatsu, T.; Kambara, T.; Betsunoh, H.; Abe, H.; Arai, K.; Shirataki, H.; et al. Increased Nrf2 expression by renal cell carcinoma is associated with postoperative chronic kidney disease and an unfavorable prognosis. Oncotarget 2018, 9, 28351–28363. [Google Scholar] [CrossRef]
- Hishikawa, A.; Hayashi, K.; Itoh, H. Transcription Factors as Therapeutic Targets in Chronic Kidney Disease. Molecules 2018, 23, 1123. [Google Scholar] [CrossRef]
- Shen, B.; Zhao, C.; Wang, Y.; Peng, Y.; Cheng, J.; Li, Z.; Wu, L.; Jin, M.; Feng, H. Aucubin inhibited lipid accumulation and oxidative stress via Nrf2/HO-1 and AMPK signalling pathways. J. Cell. Mol. Med. 2019, 23, 4063–4075. [Google Scholar] [CrossRef]
- Maruyama, A.; Mimura, J.; Itoh, K. Non-coding RNA derived from the region adjacent to the human HO-1 E2 enhancer selectively regulates HO-1 gene induction by modulating Pol II binding. Nucleic Acids Res. 2014, 42, 13599–13614. [Google Scholar] [CrossRef] [Green Version]
- Abd El-Twab, S.M.; Hussein, O.E.; Hozayen, W.G.; Bin-Jumah, M.; Mahmoud, A.M. Chicoric acid prevents methotrexate-induced kidney injury by suppressing NF-kappaB/NLRP3 inflammasome activation and up-regulating Nrf2/ARE/HO-1 signaling. Inflamm. Res. 2019, 68, 511–523. [Google Scholar] [CrossRef]
- Silva-Islas, C.A.; Maldonado, P.D. Canonical and non-canonical mechanisms of Nrf2 activation. Pharmacol. Res. 2018, 134, 92–99. [Google Scholar] [CrossRef]
- Kneeshaw, S.; Keyani, R.; Delorme-Hinoux, V.; Imrie, L.; Loake, G.J.; Le Bihan, T.; Reichheld, J.P.; Spoel, S.H. Nucleoredoxin guards against oxidative stress by protecting antioxidant enzymes. Proc. Natl. Acad. Sci. USA 2017. [Google Scholar] [CrossRef]
- Lv, H.; Liu, Q.; Wen, Z.; Feng, H.; Deng, X.; Ci, X. Xanthohumol ameliorates lipopolysaccharide (LPS)-induced acute lung injury via induction of AMPK/GSK3β-Nrf2 signal axis. Redox Biol. 2017, 12, 311–324. [Google Scholar] [CrossRef]
- Maiese, K. New Insights for Oxidative Stress and Diabetes Mellitus. Oxidative Med. Cell. Longev. 2015, 2015, 875961. [Google Scholar] [CrossRef]
- Liu, J.; Wu, J.; Sun, A.; Sun, Y.; Yu, X.; Liu, N.; Dong, S.; Yang, F.; Zhang, L.; Zhong, X.; et al. Hydrogen sulfide decreases high glucose/palmitate-induced autophagy in endothelial cells by the Nrf2-ROS-AMPK signaling pathway. Cell Biosci. 2016, 6, 33. [Google Scholar] [CrossRef]
- Lamberts, R.R.; Onderwater, G.; Hamdani, N.; Vreden, M.J.; Steenhuisen, J.; Eringa, E.C.; Loer, S.A.; Stienen, G.J.; Bouwman, R.A. Reactive oxygen species-induced stimulation of 5′AMP-activated protein kinase mediates sevoflurane-induced cardioprotection. Circulation 2009, 120, S10–S15. [Google Scholar] [CrossRef]
- Banskota, S.; Regmi, S.C.; Kim, J.A. NOX1 to NOX2 switch deactivates AMPK and induces invasive phenotype in colon cancer cells through overexpression of MMP-7. Mol. Cancer 2015, 14, 123. [Google Scholar] [CrossRef]
- Laplante, M.; Sabatini, D.M. mTOR signaling in growth control and disease. Cell 2012, 149, 274–293. [Google Scholar] [CrossRef]
- Raychaudhuri, S.K.; Raychaudhuri, S.P. mTOR Signaling Cascade in Psoriatic Disease: Double Kinase mTOR Inhibitor a Novel Therapeutic Target. Indian J. Dermatol. 2014, 59, 67–70. [Google Scholar] [CrossRef]
- Dazert, E.; Hall, M.N. mTOR signaling in disease. Curr. Opin. Cell Biol. 2011, 23, 744–755. [Google Scholar] [CrossRef]
- Carling, D. AMPK signalling in health and disease. Curr. Opin. Cell Biol. 2017, 45, 31–37. [Google Scholar] [CrossRef]
- Richter, E.A.; Ruderman, N.B. AMPK and the biochemistry of exercise: Implications for human health and disease. Biochem. J. 2009, 418, 261–275. [Google Scholar] [CrossRef]
- Viollet, B.; Horman, S.; Leclerc, J.; Lantier, L.; Foretz, M.; Billaud, M.; Giri, S.; Andreelli, F. AMPK inhibition in health and disease. Crit. Rev. Biochem. Mol. Biol. 2010, 45, 276–295. [Google Scholar] [CrossRef] [Green Version]
- Gwinn, D.M.; Shackelford, D.B.; Egan, D.F.; Mihaylova, M.M.; Mery, A.; Vasquez, D.S.; Turk, B.E.; Shaw, R.J. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol. Cell 2008, 30, 214–226. [Google Scholar] [CrossRef]
- Inoki, K.; Zhu, T.; Guan, K.L. TSC2 mediates cellular energy response to control cell growth and survival. Cell 2003, 115, 577–590. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compoundsare or are available from the authors. Cyanate was purchased from Sigma-Aldrich (St Louis, MO, USA), Item number 185086. |









© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, L.; Tian, K.; Zhang, T.; Fan, C.-H.; Zhou, P.; Zeng, D.; Zhao, S.; Li, L.-S.; Smith, H.S.; Li, J.; et al. Cyanate Induces Oxidative Stress Injury and Abnormal Lipid Metabolism in Liver through Nrf2/HO-1. Molecules 2019, 24, 3231. https://doi.org/10.3390/molecules24183231
Hu L, Tian K, Zhang T, Fan C-H, Zhou P, Zeng D, Zhao S, Li L-S, Smith HS, Li J, et al. Cyanate Induces Oxidative Stress Injury and Abnormal Lipid Metabolism in Liver through Nrf2/HO-1. Molecules. 2019; 24(18):3231. https://doi.org/10.3390/molecules24183231
Chicago/Turabian StyleHu, Ling, Kuan Tian, Tao Zhang, Chun-Hua Fan, Peng Zhou, Di Zeng, Shuang Zhao, Li-Sha Li, Hendrea Shaniqua Smith, Jing Li, and et al. 2019. "Cyanate Induces Oxidative Stress Injury and Abnormal Lipid Metabolism in Liver through Nrf2/HO-1" Molecules 24, no. 18: 3231. https://doi.org/10.3390/molecules24183231