Ophiopogon Polysaccharide Promotes the In Vitro Metabolism of Ophiopogonins by Human Gut Microbiota
Abstract
:1. Introduction
2. Results
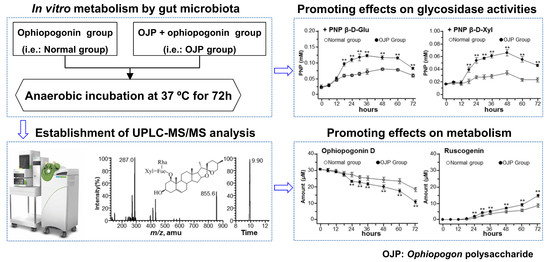
2.1. Establishment of UPLC-MS/MS Analysis
2.2. Metabolism of Ophiopogonins with Gut Microbiota
2.3. Glycosidase Activities during Fermentation with Gut Microbiota
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Human Gut Microbiota Preparation
4.3. Fermentation of Ophiopogonin with Gut Microbiota
4.4. Assay for Glycosidase Activities
4.5. Sample Preparation for UPLC-MS/MS
4.6. UPLC-MS/MS Analysis
4.7. Method Validation
4.8. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Chen, M.H.; Chen, X.J.; Wang, M.; Lin, L.G.; Wang, Y.T. Ophiopogon japonicus—A phytochemical, ethnomedicinal and pharmacological review. J. Ethnopharmacol. 2016, 181, 193–213. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Wang, X.; Lu, M.; He, X.; Yang, X. Recent advances in polysaccharides from Ophiopogon japonicus and Liriope spicata var. prolifera. Int. J. Biol. Macromol. 2018, 114, 1257–1266. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Li, X.; Liu, L.; Liu, C.; Han, X. Ophiopogonin D alleviates high-fat diet-induced metabolic syndrome and changes the structure of gut microbiota in mice. FASEB J. 2018, 32, 1139–1153. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.L.; Yuan, W.W.; Hu, Z.F.; Qi, J.; Zhu, D.N.; Yu, B.Y. Determination and pharmacokinetics of DT-13 in rat plasma by LC-MS. J. Pharm. Biomed. Anal. 2011, 56, 650–654. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Tang, Y.; Fawcett, J.P.; Gu, J.; Zhong, D. Characterization of the pharmacokinetics of dioscin in rat. Steroids 2005, 70, 525–530. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Xu, D.S.; Feng, Y.; Li, X.; Wu, X.R. Effect of intestinal bacteria on metabolism of ophiopognin saponin D’ in rats. China J. Chin. Mater. Med. 2005, 30, 618–620. [Google Scholar]
- Shen, L.; Feng, Y.; Xu, D.S.; Liu, Y.M.; Lai, Y.J. Determining metabolite diosgenin of Ophiopogon japonicus saponin in vivo by HPLC-MS. Chin. Tradit. Pat. Med. 2006, 28, 1178–1181. [Google Scholar]
- Zhou, R.N.; Song, Y.L.; Ruan, J.Q.; Wang, Y.T.; Yan, R. Pharmacokinetic evidence on the contribution of intestinal bacterial conversion to beneficial effects of astragaloside IV, a marker compound of astragali radix, in traditional oral use of the herb. Drug Metab. Pharmacokinet. 2012, 27, 586–597. [Google Scholar] [CrossRef]
- Wang, H.Y.; Hua, H.Y.; Liu, X.Y.; Liu, J.H.; Yu, B.Y. In vitro biotransformation of red ginseng extract by human intestinal microflora: Metabolites identification and metabolic profile elucidation using LC-Q-TOF/MS. J. Pharm. Biomed. Anal. 2014, 98, 296–306. [Google Scholar] [CrossRef]
- Chen, F.; Wen, Q.; Jiang, J.; Li, H.L.; Tan, Y.F.; Li, Y.H.; Zeng, N.K. Could the gut microbiota reconcile the oral bioavailability conundrum of traditional herbs? J. Ethnopharmacol. 2016, 179, 253–264. [Google Scholar] [CrossRef]
- Li, X.E.; Wang, Y.X.; Sun, P.; Liao, D.Q. Determination of Saponin Content in Hang Maidong and Chuan Maidong via HPLC-ELSD Analysis. J. Anal. Methods Chem. 2016, 2016, 7214607. [Google Scholar] [CrossRef] [PubMed]
- Commission of Chinese Pharmacopoeia. Pharmacopoeia of the People’s Republic of China; People’s Medical Publishing House: Beijing, China, 2015; Volume 1. [Google Scholar]
- HKCMMs. Hong Kong Chinese Materia Medica Standards; Department of Health, Hong Kong Special Administrative Region, The People’s Republic of China: Hong Kong, China, 2010; Volume 3.
- Gong, Y.; Zhang, J.; Gao, F.; Zhou, J.; Xiang, Z.; Zhou, C.; Wan, L.; Chen, J. Structure features and in vitro hypoglycemic activities of polysaccharides from different species of Maidong. Carbohydr. Polym. 2017, 173, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.L.; Li, Y.; Wang, Y.; Feng, Y. MDG-1, an Ophiopogon polysaccharide, regulate gut microbiota in high-fat diet-induced obese C57BL/6 mice. Int. J. Biol. Macromol. 2015, 81, 576–583. [Google Scholar] [CrossRef] [PubMed]
- Mischke, M.; Arora, T.; Tims, S.; Engels, E.; Sommer, N.; van Limpt, K.; Baars, A.; Oozeer, R.; Oosting, A.; Backhed, F.; et al. Specific synbiotics in early life protect against diet-induced obesity in adult mice. Diabetes Obes. Metab. 2018, 20, 1408–1418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.Y.; Guo, L.X.; Hu, W.H.; Peng, Z.T.; Wang, C.; Chen, Z.C.; Liu, E.Y.L.; Dong, T.X.T.; Wang, T.J.; Tsim, K.W.K. Polysaccharide from tuberous roots of Ophiopogon japonicus regulates gut microbiota and its metabolites during alleviation of high-fat diet-induced type-2 diabetes in mice. J. Funct. Foods. Under review.
- Li, J.; Li, R.; Li, N.; Zheng, F.; Dai, Y.; Ge, Y.; Yue, H.; Yu, S. Mechanism of antidiabetic and synergistic effects of ginseng polysaccharide and ginsenoside Rb1 on diabetic rat model. J. Pharm. Biomed. Anal. 2018, 158, 451–460. [Google Scholar] [CrossRef] [PubMed]
- Dabek, M.; McCrae, S.I.; Stevens, V.J.; Duncan, S.H.; Louis, P. Distribution of beta-glucosidase and beta-glucuronidase activity and of beta-glucuronidase gene gus in human colonic bacteria. FEMS Microbiol. Ecol. 2008, 66, 487–495. [Google Scholar] [CrossRef]
- Ji, P.Y.; Li, Z.W.; Yang, Q.; Wu, R. Rapid determination of ruscogenin in rat plasma with application to pharmacokinetic study. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2015, 985, 71–74. [Google Scholar] [CrossRef]
- Jiang, P.; Lu, Y.; Chen, D. Qualitative and quantitative analysis of multiple components for quality control of Deng-Zhan-Sheng-Mai capsules by ultra high performance liquid chromatography tandem mass spectrometry method coupled with chemometrics. J. Sep. Sci. 2017, 40, 612–624. [Google Scholar] [CrossRef]
- Yu, J.; Xin, Y.F.; Gu, L.Q.; Gao, H.Y.; Xia, L.J.; You, Z.Q.; Xie, F.; Ma, Z.F.; Wang, Z.; Xuan, Y.X. One-month toxicokinetic study of SHENMAI injection in rats. J. Ethnopharmacol. 2014, 154, 391–399. [Google Scholar] [CrossRef]
- Xu, L.; Liu, Y.; Wang, T.; Qi, Y.; Han, X.; Xu, Y.; Peng, J.; Tang, X. Development and validation of a sensitive and rapid non-aqueous LC-ESI-MS/MS method for measurement of diosgenin in the plasma of normal and hyperlipidemic rats: A comparative study. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2009, 877, 1530–1536. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Xian, Y.; Xiao, K.; Wu, Y.; Zhu, L.; He, J. Development and comparison of single-step solid phase extraction and QuEChERS clean-up for the analysis of 7 mycotoxins in fruits and vegetables during storage by UHPLC-MS/MS. Food Chem. 2019, 274, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Xia, C.; Wang, G.; Sun, J.; Hao, H.; Xiong, Y.; Gu, S.; Shang, L.; Zheng, C. Simultaneous determination of ginsenoside Rg1, Re, Rd, Rb1 and ophiopogonin D in rat plasma by liquid chromatography/electrospray ionization mass spectrometric method and its application to pharmacokinetic study of ‘SHENMAI’ injection. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2008, 862, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Xia, C.H.; Sun, J.G.; Hao, H.P.; Wang, G.J.; Yan, B.; Gu, S.H.; Zheng, C.N.; Shang, L.L.; Roberts, M.S. Quantitative determination of ophiopogonin d by liquid chromatography/electrospray ionization mass spectrometry and its pharmacokinetics in rat. Planta Med. 2008, 74, 1832–1836. [Google Scholar] [CrossRef] [PubMed]
- Zhan, S.; Shao, Q.; Fan, X.; Li, Z. Development of a sensitive LC-MS/MS method for simultaneous quantification of eleven constituents in rat serum and its application to a pharmacokinetic study of a Chinese medicine Shengmai injection. Biomed. Chromatogr. 2015, 29, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Reimer, R.A.; Maathuis, A.J.; Venema, K.; Lyon, M.R.; Gahler, R.J.; Wood, S. Effect of the novel polysaccharide PolyGlycopleX(R) on short-chain fatty acid production in a computer-controlled in vitro model of the human large intestine. Nutrients 2014, 6, 1115–1127. [Google Scholar] [CrossRef] [PubMed]
- Van den Abbeele, P.; Venema, K.; Van de Wiele, T.; Verstraete, W.; Possemiers, S. Different human gut models reveal the distinct fermentation patterns of Arabinoxylan versus inulin. J. Agric. Food Chem. 2013, 61, 9819–9827. [Google Scholar] [CrossRef] [PubMed]
- Du, L.Y.; Zhao, M.; Xu, J.; Qian, D.W.; Jiang, S.; Shang, E.X.; Guo, J.M.; Duan, J.A. Analysis of the metabolites of isorhamnetin 3-O-glucoside produced by human intestinal flora in vitro by applying ultraperformance liquid chromatography/quadrupole time-of-flight mass spectrometry. J. Agric. Food Chem. 2014, 62, 2489–2495. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.A.; Jung, I.H.; Park, S.H.; Ahn, Y.T.; Huh, C.S.; Kim, D.H. Comparative analysis of the gut microbiota in people with different levels of ginsenoside Rb1 degradation to compound K. PLoS ONE 2013, 8, e62409. [Google Scholar] [CrossRef]
- Kim, K.A.; Yoo, H.H.; Gu, W.; Yu, D.H.; Jin, M.J.; Choi, H.L.; Yuan, K.; Guerin-Deremaux, L.; Kim, D.H. A prebiotic fiber increases the formation and subsequent absorption of compound K following oral administration of ginseng in rats. J. Ginseng Res. 2015, 39, 183–187. [Google Scholar] [CrossRef]
- Choi, J.R.; Hong, S.W.; Kim, Y.; Jang, S.E.; Kim, N.J.; Han, M.J.; Kim, D.H. Metabolic activities of ginseng and its constituents, ginsenoside rb1 and rg1, by human intestinal microflora. J. Ginseng Res. 2011, 35, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Gao, X.J.; Li, T.; Jing, W.H.; Han, B.L.; Jia, Y.M.; Hu, N.; Yan, Z.X.; Li, S.L.; Yan, R. Ginseng polysaccharides enhanced ginsenoside Rb1 and microbial metabolites exposure through enhancing intestinal absorption and affecting gut microbial metabolism. J. Ethnopharmacol. 2018, 216, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Yao, R.; Wong, C.B.; Nakamura, K.; Mitsuyama, E.; Tanaka, A.; Kuhara, T.; Odamaki, T.; Xiao, J.Z. Bifidobacterium breve MCC1274 with glycosidic activity enhances in vivo isoflavone bioavailability. Benef. Microbes 2019, 10, 521–531. [Google Scholar] [CrossRef] [PubMed]
- Truchado, P.; Van den Abbeele, P.; Riviere, A.; Possemiers, S.; De Vuyst, L.; Van de Wiele, T. Bifidobacterium longum D2 enhances microbial degradation of long-chain arabinoxylans in an in vitro model of the proximal colon. Benef. Microbes 2015, 6, 849–860. [Google Scholar] [CrossRef] [PubMed]
- Amaretti, A.; Raimondi, S.; Leonardi, A.; Quartieri, A.; Rossi, M. Hydrolysis of the rutinose-conjugates flavonoids rutin and hesperidin by the gut microbiota and bifidobacteria. Nutrients 2015, 7, 2788–2800. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Hou, Q.; Zhang, J.; Xu, H.; Sun, Z.; Menghe, B.; Zhang, H. Carbohydrate Staple Food Modulates Gut Microbiota of Mongolians in China. Front. Microbiol. 2017, 8, 484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeo, H.K.; Hyun, Y.J.; Jang, S.E.; Han, M.J.; Lee, Y.S.; Kim, D.H. Development of fecal microbial enzyme mix for mutagenicity assay of natural products. J. Microbiol. Biotechnol. 2012, 22, 838–848. [Google Scholar] [CrossRef] [PubMed]
- Ju, Z.; Li, J.; Lu, Q.; Yang, Y.; Yang, L.; Wang, Z. Identification and quantitative investigation of the effects of intestinal microflora on the metabolism and pharmacokinetics of notoginsenoside Fc assayed by liquid chromatography with electrospray ionization tandem mass spectrometry. J. Sep. Sci. 2019, 42, 1740–1749. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Qian, D.; Jiang, S.; Shang, E.X.; Guo, J.; Duan, J.A. Identification of rutin deglycosylated metabolites produced by human intestinal bacteria using UPLC-Q-TOF/MS. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2012, 898, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Guidance for Industry: Bioanalytical Method Validation; U.S. Department of Health and Human Services, Food and Drug Administration, CDER: Rockville, MD, USA, 2013.
Sample Availability: Samples of the Ophiopogon polysaccharide and ophiopogonin compounds in this work are available from the authors. |



| No. | Rt (min) | Analyte | Mw | Precursor Ion | Product Ion | Entrance Voltage (V) | Collision Energy (eV) |
|---|---|---|---|---|---|---|---|
| 1 | 9.90 | Ophiopogonin D | 855.02 | 855.6 | 287.0 | 20 | −55 |
| 2 | 9.99 | Ophiopogonin D’ | 855.02 | 855.6 | 253.0 | 20 | −72 |
| 3 | 10.91 | Ruscogenin | 430.62 | 431.3 | 287.0 | 20 | −24 |
| 4 | 11.95 | Diosgenin | 414.62 | 415.2 | 271.0 | 20 | −23 |
| IS | 4.23 | Digoxin | 780.94 | 803.5 | 387.0 | 20 | −26 |
| Analyte | Calibration Curve | R2a | Linear Range (μM) | LLOQ b (μM) | LOD c (μM) |
|---|---|---|---|---|---|
| Ophiopogonin D | y = 0.4461x − 0.0870 | 0.9986 | 0.24–60 | 0.24 | 0.07 |
| Ophiopogonin D’ | y = 0.4688x − 0.0442 | 0.9976 | 0.24–60 | 0.24 | 0.07 |
| Ruscogenin | y = 0.3848x + 0.1414 | 0.9985 | 0.24–60 | 0.24 | 0.07 |
| Diosgenin | y = 0.3767x + 0.1790 | 0.9977 | 0.24–60 | 0.24 | 0.07 |
| Analyte | Spiked (μM) | Intra-Day (n = 5) | Inter-Day (n = 15) | ||||
|---|---|---|---|---|---|---|---|
| Measured (μM) | Precision (RSD%) | Accuracy (RE%) | Measured (μM) | Precision (RSD%) | Accuracy (RE%) | ||
| Ophiopogonin D | 0.60 | 0.62 | 3.80 | 3.43 | 0.59 | 11.87 | −1.79 |
| 3.00 | 2.89 | 8.09 | −3.61 | 3.06 | 13.63 | 2.10 | |
| 30.0 | 29.2 | 10.07 | −2.57 | 31.6 | 10.31 | 5.43 | |
| Ophiopogonin D’ | 0.6 | 0.59 | 12.05 | −2.25 | 0.65 | 9.46 | 8.79 |
| 3.0 | 3.11 | 12.88 | 3.60 | 3.24 | 11.51 | 7.91 | |
| 30.0 | 30.2 | 14.87 | 0.68 | 31.9 | 8.09 | 6.30 | |
| Ruscogenin | 0.6 | 0.65 | 5.95 | 9.12 | 0.65 | 3.96 | 7.94 |
| 3.0 | 3.20 | 4.94 | 6.68 | 3.20 | 6.36 | 6.91 | |
| 30.0 | 31.8 | 12.39 | 6.17 | 33.4 | 12.67 | 11.51 | |
| Diosgenin | 0.6 | 0.65 | 6.14 | 8.95 | 0.62 | 8.68 | 4.51 |
| 3.0 | 3.13 | 3.74 | 4.43 | 3.07 | 8.78 | 2.40 | |
| 30.0 | 30.19 | 8.04 | 0.62 | 31.6 | 8.45 | 5.19 | |
| Analyte | Spiked (μM) | Extraction Efficiency (n = 5) | Matrix Effects (n = 5) | Autosampler Stability (n = 6) | |||
|---|---|---|---|---|---|---|---|
| Measured (%) | RSD (%) | Measured (%) | RSD (%) | Measured (%) | RSD (%) | ||
| Ophiopogonin D | 0.60 | 94.74 | 7.85 | 97.85 | 9.85 | 102.91 | 8.58 |
| 3.00 | 96.17 | 6.36 | 87.83 | 9.08 | 94.33 | 5.54 | |
| 30.0 | 104.61 | 13.05 | 89.92 | 5.81 | 99.13 | 12.64 | |
| Ophiopogonin D’ | 0.60 | 88.86 | 8.75 | 96.78 | 12.76 | 89.69 | 7.36 |
| 3.00 | 96.13 | 10.97 | 94.36 | 6.5 | 97.15 | 8.17 | |
| 30.0 | 98.66 | 10.34 | 96.89 | 6.95 | 101.59 | 11.39 | |
| Ruscogenin | 0.60 | 86.79 | 5.27 | 87.93 | 6.96 | 102.68 | 4.87 |
| 3.00 | 94.54 | 5.7 | 92.28 | 6.98 | 96.95 | 6.67 | |
| 30.0 | 99.32 | 3.46 | 97.06 | 6.11 | 94.70 | 6.58 | |
| Diosgenin | 0.60 | 90.17 | 9.08 | 92.29 | 7.89 | 102.60 | 6.52 |
| 3.00 | 86.50 | 5.72 | 94.90 | 7.39 | 97.04 | 3.65 | |
| 30.0 | 96.76 | 4.19 | 97.10 | 5.74 | 92.17 | 4.52 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.-Y.; Guo, S.-C.; Peng, Z.-T.; Wang, C.; Duan, R.; Dong, T.T.X.; Tsim, K.W.K. Ophiopogon Polysaccharide Promotes the In Vitro Metabolism of Ophiopogonins by Human Gut Microbiota. Molecules 2019, 24, 2886. https://doi.org/10.3390/molecules24162886
Wang H-Y, Guo S-C, Peng Z-T, Wang C, Duan R, Dong TTX, Tsim KWK. Ophiopogon Polysaccharide Promotes the In Vitro Metabolism of Ophiopogonins by Human Gut Microbiota. Molecules. 2019; 24(16):2886. https://doi.org/10.3390/molecules24162886
Chicago/Turabian StyleWang, Huai-You, Shu-Chen Guo, Zhi-Tian Peng, Cheng Wang, Ran Duan, Tina T. X. Dong, and Karl W. K. Tsim. 2019. "Ophiopogon Polysaccharide Promotes the In Vitro Metabolism of Ophiopogonins by Human Gut Microbiota" Molecules 24, no. 16: 2886. https://doi.org/10.3390/molecules24162886