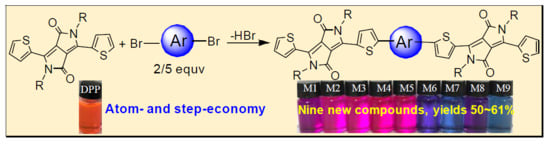
Novel Diketopyrrolopyrrole-Based π-Conjugated Molecules Synthesized Via One-Pot Direct Arylation Reaction
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Characterizations
3.3. General Synthetic Procedure
3.4. Characterization Data of All Products
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lin, Y.; Zhan, X. Oligomer Molecules for Efficient Organic Photovoltaics. Acc. Chem. Res. 2016, 49, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Shirakawa, H.; Louis, E.J.; MacDiarmid, A.G.; Chiang, C.K.; Heeger, A.J. Synthesis of electrically conducting organic polymers: Halogen derivatives of polyacetylene, (CH)x. J. Chem. Soc. Chem. Commun. 1977, 16, 578–580. [Google Scholar] [CrossRef]
- Cai, Z.; Lo, W.-Y.; Zheng, T.; Li, L.; Zhang, N.; Hu, Y.; Yu, L. Exceptional Single-Molecule Transport Properties of Ladder-Type Heteroacene Molecular Wires. J. Am. Chem. Soc. 2016, 138, 10630–10635. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.Y.; Cheng, J.Z.; Zhang, X.-F.; Liu, H.; Shen, Z.-Q.; Wen, H.-R. Single-step access to a series of D–A π-conjugated oligomers with 3–10 nm chain lengths. Polym. Chem. 2019, 10, 325–330. [Google Scholar] [CrossRef]
- Farnum, D.G.; Mehta, G.; Moore, G.G.I.; Siega, F.P. Attempted Reformatskii reaction of benzonitrile, 1,4-diketo-3,6-diphenylpyrrolo[3,4-C] pyrrole. A lactam analogue of pentalene. Tetrahedron Lett. 1974, 29, 2549–2552. [Google Scholar] [CrossRef]
- He, C.-Y.; Wang, Z.; Wu, C.-Z.; Qing, F.-L.; Zhang, X. Pd-catalyzed oxidative cross-coupling between two electron-rich heteroarenes. Chem. Sci. 2013, 4, 3508–3513. [Google Scholar] [CrossRef]
- Walker, B.; Tamayo, A.B.; Dang, X.D.; Zalar, P.; Seo, J.-H.; Garcia, A.; Tantiwiwat, M.; Nguyen, T.Q. Nanoscale Phase Separation and High Photovoltaic Efficiency in Solution-Processed, Small-Molecule Bulk Heterojunction Solar Cells. Adv. Funct. Mater. 2009, 19, 3063–3069. [Google Scholar] [CrossRef]
- Pouliot, J.-R.; Sun, B.; Leduc, M.; Najari, A.; Li, Y.; Leclerc, M. A high mobility DPP-based polymer obtained via direct (hetero)arylation polymerization. Polym. Chem. 2015, 6, 278–282. [Google Scholar] [CrossRef]
- Sonar, P.; Foong, T.R.B.; Dodabalapur, A. Synthesis of diketopyrrolopyrrole based copolymers via the direct arylation method for p-channel and ambipolar OFETs. Phys. Chem. Chem. Phys. 2014, 16, 4275–4283. [Google Scholar] [CrossRef]
- Bura, T.; Beaupré, S.; Légaré, M.-A.; Ibraikulov, O.A.; Leclerc, N.; Leclerc, M. Theoretical Calculations for Highly Selective Direct Heteroarylation Polymerization: New Nitrile-Substituted Dithienyl-Diketopyrrolopyrrole-Based Polymers. Molecules 2018, 23, 2324. [Google Scholar] [CrossRef]
- Welsh, T.A.; Laventure, A.; Welch, G.C. Direct (Hetero)Arylation for the Synthesis of Molecular Materials: Coupling Thieno [3,4-c] pyrrole-4,6-dione with Perylene Diimide to Yield Novel Non-Fullerene Acceptors for Organic Solar Cells. Molecules 2018, 23, 931. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Xu, B.; Xiang, M.; Chen, X.; Wang, Y.; Liu, Z. Catalytic Performance of Nitrogen-Doped Activated Carbon Supported Pd Catalyst for Hydrodechlorination of 2,4-Dichlorophenol or Chloropentafluoroethane. Molecules 2019, 24, 4674. [Google Scholar] [CrossRef] [PubMed]
- Tabasso, S.; Gaudino, E.C.; Acciardo, E.; Manzoli, M.; Giacomino, A.; Cravotto, G. Microwave-Assisted Dehydrogenative Cross Coupling Reactions in γ-valerolactone with a Reusable Pd/β-cyclodextrin Crosslinked Catalyst. Molecules 2019, 24, 2288. [Google Scholar] [CrossRef] [PubMed]
- Wakioka, M.; Yamashita, N.; Mori, H.; Nishihara, Y.; Ozawa, F. Synthesis of a 1,2-Dithienylethene-Containing Donor-Acceptor Polymer via Palladium-Catalyzed Direct Arylation Polymerization (DArP). Molecules 2018, 23, 4981. [Google Scholar] [CrossRef]
- Obora, Y.; Ishii, Y. Pd(II)/HPMoV-Catalyzed Direct Oxidative Coupling Reaction of Benzenes with Olefins. Molecules 2010, 15, 1487–1500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, C.; You, L.; Chen, C. Palladium-Catalyzed C–H Arylation of 1,2,3-Triazoles. Molecules 2016, 21, 1268. [Google Scholar] [CrossRef]
- Lafrance, M.; Rowley, C.N.; Woo, T.K.; Fagnou, K. Catalytic Intermolecular Direct Arylation of Perfluorobenzenes. J. Am. Chem. Soc. 2006, 128, 8754–8756. [Google Scholar] [CrossRef] [Green Version]
- Lawrence, J.; Goto, E.; Ren, J.M.; McDearmon, B.; Kim, D.S.; Ochiai, Y. A Versatile and Efficient Strategy to Discrete Conjugated Oligomers. J. Am. Chem. Soc. 2017, 139, 13735–13739. [Google Scholar] [CrossRef]
- Maria, F.D.; Olivelli, P.; Gazzano, M.; Zanelli, A.; Biasiucci, M.; Gigli, G. A Successful Chemical Strategy to Induce Oligothiophene Self-Assembly into Fibers with Tunable Shape and Function. J. Am. Chem. Soc. 2011, 133, 8654–8661. [Google Scholar] [CrossRef]
- Wang, Q.; Qu, Y.; Tian, H.; Geng, Y.; Wang, F. Iterative Binomial Synthesis of Monodisperse Polyfluorenes up to 64-mers and Their Chain-Length-Dependent Properties. Macromolecules 2011, 44, 1256–1260. [Google Scholar] [CrossRef]
- Burckstummer, H.; Weissenstein, A.; Bialas, D.; Wurthner, F. Colorimetric and Ratiometric Fluorescent Chemosensor Based on Diketopyrrolopyrrole for Selective Detection of Thiols: An Experimental and Theoretical Study. J. Org. Chem. 2011, 76, 2426–2432. [Google Scholar]
- Kylberg, W.; Sonar, P.; Heier, J.; Tisserant, J.-N.; Muller, C.; Nuesch, F.; Chen, Z.-K.; Dodabalapur, A.; Yoon, S.; Hany, R. Synthesis, thin-film morphology, and comparative study of bulk and bilayer heterojunction organic photovoltaic devices using soluble diketopyrrolopyrrole molecules. Energy Environ. Sci. 2011, 4, 3617–3624. [Google Scholar] [CrossRef]
- Chávez, P.; Bulut, I.; Fall, S.; Ibraikulov, O.A.; Chochos, C.L.; Bartringer, J.; Heiser, T.; Lévêque, P.; Leclerc, N. An Electron-Transporting Thiazole-Based Polymer Synthesized Through Direct (Hetero)Arylation Polymerization. Molecules 2018, 23, 1270. [Google Scholar] [CrossRef]
- Kalepu, J.; Pilarski, T.L. Weinreb Amides as Directing Groups for Transition Metal-Catalyzed C–H Functionalizations. Molecules 2019, 24, 830. [Google Scholar] [CrossRef]
- Pouliot, J.-R.; Grenier, F.; Blaskovits, J.T.; Beaupre, S.; Leclerc, M. Direct (Hetero)arylation Polymerization: Simplicity for Conjugated Polymer Synthesis. Chem. Rev. 2016, 116, 14225–14274. [Google Scholar] [CrossRef]
- Josse, P.; Dayneko, S.; Zhang, Y.; Seignon, S.D.; Zhang, S.; Blanchard, P.; Welch, G.C.; Cabanetos, C. Direct (Hetero)Arylation Polymerization of a Spirobifluorene and a Dithienyl-Diketopyrrolopyrrole Derivative: New Donor Polymers for Organic Solar Cells. Molecules 2018, 23, 962. [Google Scholar] [CrossRef]
- Nitti, A.; Po, R.; Bianchi, G.; Pasini, D. Direct Arylation Strategies in the Synthesis of π-Extended Monomers for Organic Polymeric Solar Cells. Molecules 2017, 22, 21. [Google Scholar] [CrossRef]
- Liu, F.; Zhang, Y.; Wang, H.; Zhang, S. Novel Conjugated Polymers Prepared by Direct (Hetero) arylation: An Eco-Friendly Tool for Organic Electronics. Molecules 2018, 23, 408. [Google Scholar] [CrossRef]
- Hendsbee, A.D.; Li, Y. Performance Comparisons of Polymer Semiconductors Synthesized by Direct (Hetero)Arylation Polymerization (DHAP) and Conventional Methods for Organic Thin Film Transistors and Organic Photovoltaics. Molecules 2018, 23, 1255. [Google Scholar] [CrossRef]
- Liu, S.-Y.; Shi, M.-M.; Huang, J.-C.; Jin, Z.-N.; Hu, X.-L.; Pan, J.-Y.; Li, H.-Y.; Jen, A.K.-Y.; Chen, H.-Z. C–H activation: Making diketopyrrolopyrrole derivatives easily accessible. J. Mater. Chem. A 2013, 1, 2795–2805. [Google Scholar] [CrossRef]
- Zhang, J.; Kang, L.J.; Parker, T.C.; Blakey, S.B.; Luscombe, C.K.; Marder, S.R. Recent Developments in C–H Activation for Materials Science in the Center for Selective C–H Activation. Molecules 2018, 23, 922. [Google Scholar] [CrossRef]
- Liu, S.-Y.; Liu, H.; Shen, Z.-Q.; Huang, W.-Y.; Zhong, A.-G.; Wen, H.R. Atom-and step-economic synthesis of π-conjugated large oligomers via C–H activated oligomerization. Dye. Pigment. 2019, 162, 640–646. [Google Scholar] [CrossRef]
- Liu, S.-Y.; Wang, D.-G.; Zhong, A.-G.; Wen, H.-R. One-step rapid synthesis of π-conjugated large oligomers via C–H activation coupling. Org. Chem. Front. 2018, 5, 653–661. [Google Scholar] [CrossRef]
- Lafrance, M.; Fagnou, K. Palladium-catalyzed benzene arylation: Incorporation of catalytic pivalic acid as a proton shuttle and a key element in catalyst design. J. Am. Chem. Soc. 2006, 128, 16496–16497. [Google Scholar] [CrossRef]
- Lapointe, D.; Fagnou, K. Overview of the mechanistic work on the concerted metallation–deprotonation pathway. Chem. Lett. 2010, 39, 1118–1126. [Google Scholar] [CrossRef]
- Sprick, R.S.; Bonillo, B.; Sachs, M.; Clowes, R.; Durrant, J.R.; Adamsa, D.J.; Cooper, I.A. Extended conjugated microporous polymers for photocatalytic hydrogen evolution from water. Chem. Commun. 2016, 52, 10008–10011. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Zhan, L.; Liu, F.; Ren, J.; Shi, M.; Li, C.-Z.; Russell, T.P.; Chen, H. An Unfused-Core-Based Nonfullerene Acceptor Enables High-Efficiency Organic Solar Cells with Excellent Morphological Stability at High Temperatures. Adv. Mater. 2017, 30, 1705208. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds Ms1~9 are all available from the authors. |



 |
 |
| Molecules | λmaxs (nm) | λmaxf (nm) | LUMO (eV) | λonsets (nm) | λonsetf (nm) | Egopt (eV) | HOMO (eV) |
|---|---|---|---|---|---|---|---|
| M1 | 578.0 | 575.0 | −3.94 | 649 | 800 | 1.55 | −5.49 |
| M2 | 580.0 | 565.5 | −3.99 | 649 | 760 | 1.63 | −5.62 |
| M3 | 576.5 | 567.5 | −3.95 | 649 | 800 | 1.55 | −5.50 |
| M4 | 576.5 | 565.5 | −3.94 | 649 | 800 | 1.55 | −5.49 |
| M5 | 576.5 | 566.5 | −3.95 | 649 | 800 | 1.55 | −5.50 |
| M6 | 600.0 | 609.5 | −3.93 | 685 | 850 | 1.46 | −5.39 |
| M7 | 603.5 | 609.5 | −3.95 | 800 | 790 | 1.57 | −5.52 |
| M8 | 578.5 | 611.0 | −3.97 | 632 | 850 | 1.46 | −5.43 |
| M9 | 607.0 | 629.5 | −3.95 | 700 | 850 | 1.46 | −5.41 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.; Zhang, X.-F.; Cheng, J.-Z.; Zhong, A.-G.; Wen, H.-R.; Liu, S.-Y. Novel Diketopyrrolopyrrole-Based π-Conjugated Molecules Synthesized Via One-Pot Direct Arylation Reaction. Molecules 2019, 24, 1760. https://doi.org/10.3390/molecules24091760
Liu H, Zhang X-F, Cheng J-Z, Zhong A-G, Wen H-R, Liu S-Y. Novel Diketopyrrolopyrrole-Based π-Conjugated Molecules Synthesized Via One-Pot Direct Arylation Reaction. Molecules. 2019; 24(9):1760. https://doi.org/10.3390/molecules24091760
Chicago/Turabian StyleLiu, Hui, Xiao-Feng Zhang, Jing-Zhao Cheng, Ai-Guo Zhong, He-Rui Wen, and Shi-Yong Liu. 2019. "Novel Diketopyrrolopyrrole-Based π-Conjugated Molecules Synthesized Via One-Pot Direct Arylation Reaction" Molecules 24, no. 9: 1760. https://doi.org/10.3390/molecules24091760