Poly (Octadecyl Methacrylate-Co-Trimethylolpropane Trimethacrylate) Monolithic Column for Hydrophobic in-Tube Solid-Phase Microextraction of Chlorophenoxy Acid Herbicides
Abstract
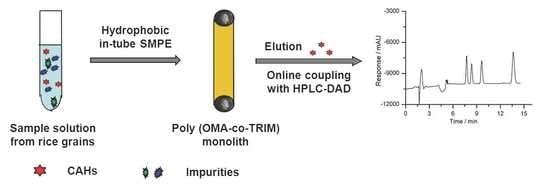
:1. Introduction
2. Results and Discussion
2.1. Characterization of the Monolithic Column for Hydrophobic in-Tube SPME
2.1.1. Morphology
2.1.2. Permeability and Porosity
2.1.3. Loading Capacity
2.1.4. Renewability
2.2. Optimization of Some Important Parameters for Hydrophobic in-Tube SPME
2.2.1. Length of the Monolithic Column
2.2.2. ACN Percentage in Sampling Solution
2.2.3. Trifluoroacetic Acid Percentage in Sampling Solution
2.2.4. Elution Volume
2.2.5. Elution Flow Rate
2.3. Method Validation
2.4. Reproducibility
2.5. Comparison of the Proposed Method with the Standard Method of LC-MS
3. Materials and Methods
3.1. Chemicals and Materials
3.2. Apparatus
3.3. Preparation of the Poly (OMA-co-TRIM) Monolithic Column
3.4. Rice Cultivation
3.5. Sample Preparation
3.6. Online Hydrophobic in-Tube SPME-HPLC System
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Krizek, B.A. Making bigger plants: Key regulators of final organ size. Curr. Opin. Plant Biol. 2009, 12, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Loomis, D.; Guyton, K.; Grosse, Y.; El Ghissasi, F.; Bouvard, V.; Benbrahim-Tallaa, L.; Guha, N.; Mattock, H.; Straif, K. Carcinogenicity of lindane, DDT, and 2,4-dichlorophenoxyacetic acid. Lancet Oncol. 2015, 16, 891–892. [Google Scholar] [CrossRef]
- National Institute for Occupational Safety and Health. The effects of workplace hazards on male reproductive health. Available online: https://www.cdc.gov/niosh/docs/96-132/ (accessed on 27 March 2019).
- Hou, X.; Han, M.; Dai, X.H.; Yang, X.F.; Yi, S.G. A multi-residue method for the determination of 124 pesticides in rice by modified QuEChERS extraction and gas chromatography-tandem mass spectrometry. Food Chem. 2013, 138, 1198–1205. [Google Scholar] [CrossRef] [PubMed]
- Nie, J.; Chen, F.; Song, Z.; Sun, C.; Li, Z.; Liu, W.; Lee, M. Large volume of water samples introduced in dispersive liquid-liquid microextraction for the determination of 15 triazole fungicides by gas chromatography-tandem mass spectrometry. Anal. Bioanal. Chem. 2016, 408, 7461–7471. [Google Scholar] [CrossRef]
- Yeh, M.K.; Lin, S.L.; Leong, M.I. Determination of phenoxyacetic acids and chlorophenols in aqueous samples by dynamic liquid-liquid-liquid microextraction with ion-pair liquid chromatography. Anal. Sci. 2011, 27, 49–54. [Google Scholar] [CrossRef]
- Shamsipur, M.; Fattahi, N.; Pirsaheb, M. Simultaneous preconcentration and determination of 2,4-D, alachlor and atrazine in aqueous samples using dispersive liquid–liquid microextraction followed by high-performance liquid chromatography ultraviolet detection. J. Sep. Sci. 2012, 35, 2718–2724. [Google Scholar] [CrossRef]
- Kittlaus, S.; Schimanke, J.; Kempe, G.; Speer, K. Development and validation of an efficient automated method for the analysis of 300 pesticides in foods using two-dimensional liquid chromatography–tandem mass spectrometry. J. Chromatogr. A 2013, 1283, 98–109. [Google Scholar] [CrossRef]
- Xu, Y.; Kutsanedzie, F.Y.H.; Hassan, M.M.; Li, H.; Chen, Q. Synthesized Au NPs@silica composite as surface-enhanced Raman spectroscopy (SERS) substrate for fast sensing trace contaminant in milk. Spectrochim. Acta A 2019, 206, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Tabani, H.; Fakhari, A.R.; Zand, E. Low-voltage electromembrane extraction combined with cyclodextrin modified capillary electrophoresis for the determination of phenoxy acid herbicides in environmental samples. Anal. Methods 2013, 5, 1548–1555. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiao, B. Dispersive liquid–liquid microextraction combined with online preconcentration MEKC for the determination of some phenoxyacetic acids in drinking water. J. Sep. Sci. 2013, 36, 3067–3074. [Google Scholar] [CrossRef]
- Deng, A.P.; Yang, H. A multichannel electrochemical detector coupled with an ELISA microtiter plate for the immunoassay of 2,4-dichlorophenoxyacetic acid. Sens. Actuators B 2007, 124, 202–208. [Google Scholar] [CrossRef]
- National Standards of People’s Republic of China. GB/T 5009 175-2003: Determination of 2,4-D in grains and vegetables. Available online: http://down.foodmate.net/standard/sort/3/2853.html (accessed on 27 March 2019).
- Agriculture standards of the People’s Republic of China. NY/T 1434-2007: Multi-residue determination of 2,4-D and other 12 Herbicides by LC/MS, 2007. Available online: http://down.foodmate.net/standard/sort/5/14681.html (accessed on 27 March 2019).
- Gure, A.; Megersa, N.; Retta, N. Ion-pair assisted liquid-liquid extraction for selective separation and analysis of multiclass pesticide residues in environmental waters. Anal. Methods 2014, 6, 4633–4642. [Google Scholar] [CrossRef]
- David, M.D.; Campbell, S.; Li, Q.X. Pressurized Fluid Extraction of Nonpolar Pesticides and Polar Herbicides Using In Situ Derivatization. Anal. Chem. 2000, 72, 3665–3670. [Google Scholar] [CrossRef]
- Moret, S.; Sánchez, J.M.; Salvadó, V.; Hidalgo, M. The evaluation of different sorbents for the preconcentration of phenoxyacetic acid herbicides and their metabolites from soils. J. Chromatogr. A 2005, 1099, 55–63. [Google Scholar] [CrossRef]
- Barchanska, H.; Danek, M.; Sajdak, M.; Turek, M. Review of sample preparation techniques for the analysis of selected classes of pesticides in plant matrices. Crit. Rev. Anal. Chem. 2018, 48, 467–491. [Google Scholar] [CrossRef]
- Hassan, J.; Shamsipur, M.; Es’haghi, A.; Fazili, S. Determination of chlorophenoxy acid herbicides in water samples by suspended liquid-phase microextraction–liquid chromatography. Chromatographia 2011, 73, 999–1003. [Google Scholar] [CrossRef]
- Yun, Y.H.; Shon, H.K.; Yoon, S.D. Preparation and characterization of molecularly imprinted polymers for the selective separation of 2,4-dichlorophenoxyacetic acid. J. Mater. Sci. 2009, 44, 6206–6211. [Google Scholar] [CrossRef]
- Zaidi, S.A. Recent developments in molecularly imprinted polymer nanofibers and their applications. Anal. Methods 2015, 7, 7406–7415. [Google Scholar] [CrossRef]
- Zhang, S.; Xu, T.; Liu, Q.; Liu, J.; Lu, F.; Yue, M.; Li, Y.; Sun, Z.; You, J. Cationic gemini surfactant-resorcinol-aldehyde resin and its application in the extraction of endocrine disrupting compounds from food contacting materials. Food Chem. 2019, 277, 404–413. [Google Scholar] [CrossRef]
- Chu, X.G.; Hu, X.Z.; Yao, H.Y. Determination of 266 pesticide residues in apple juice by matrix solid-phase dispersion and gas chromatography–mass selective detection. J. Chromatogr. A 2005, 1063, 201–210. [Google Scholar] [CrossRef] [Green Version]
- Hogendoorn, E.A.; Huls, R.; Dijkman, E. Microwave assisted solvent extraction and coupled-column reversed-phase liquid chromatography with UV detection: Use of an analytical restricted-access-medium column for the efficient multi-residue analysis of acidic pesticides in soils. J. Chromatogr. A 2001, 938, 23–33. [Google Scholar] [CrossRef]
- Fernandez-Alvarez, M.; Llompart, M.; Lamas, J.P.; Lores, M.; Garcia-Jares, C.; Garcia-Chao, M.; Dagnac, T. Simultaneous extraction and cleanup method based on pressurized solvent extraction for multiresidue analysis of pesticides in complex feed samples. J. Agric. Food Chem. 2009, 57, 3963–3973. [Google Scholar] [CrossRef]
- Wang, T.; Chen, Y.; Ma, J.; Qian, Q.; Jin, Z.; Zhang, L.; Zhang, Y. Attapulgite nanoparticles-modified monolithic column for hydrophilic in-tube solid-phase microextraction of cyromazine and melamine. Anal. Chem. 2016, 88, 1535–1541. [Google Scholar] [CrossRef]
- Beloti, L.G.M.; Miranda, L.F.C.; Queiroz, M.E.C. Butyl methacrylate-co-ethylene glycol dimethacrylate monolith for online in-tube SPME-UHPLC-MS/MS to determine chlopromazine, clozapine, quetiapine, olanzapine, and their metabolites in plasma samples. Molecules 2019, 24, 310. [Google Scholar] [CrossRef]
- Pei, M.; Shi, X.; Wu, J.; Huang, X. Graphene reinforced multiple monolithic fiber solid-phase microextraction of phenoxyacetic acid herbicides in complex samples. Talanta 2019, 191, 257–264. [Google Scholar] [CrossRef]
- Wang, J.; Wu, F.; Zhao, Q. Synchronous extraction and determination of phenoxy acid herbicides in water by on-line monolithic solid phase microextraction-high performance liquid chromatography. Chin. J. Chromatogr. 2015, 33, 849–855. [Google Scholar] [CrossRef]
- Gusev, I.; Huang, X.; Horvath, C. Capillary columns with in situ formed porous monolithic packing for micro high-performance liquid chromatography and capillary electrochromatography. J. Chromatogr. A 1999, 855, 273–290. [Google Scholar] [CrossRef]
- Wang, F.; Dong, J.; Jiang, X.; Ye, M.; Zou, H. Capillary trap column with strong cation-exchange monolith for automated shotgun proteome analysis. Anal. Chem. 2007, 79, 6599–6606. [Google Scholar] [CrossRef]
- Zhang, W.; Zhou, W.; Chen, Z. Graphene/polydopamine-modified polytetrafluoroethylene microtube for the sensitive determination of three active components in Fructus Psoraleae by online solid-phase microextraction with high-performance liquid chromatography. J. Sep. Sci. 2014, 37, 3110–3116. [Google Scholar] [CrossRef]
- Commission Regulation (EU) No 1317/2013, Amending Annexes II, III and V to Regulation (EC) No 396/2005 of the European Parliament and of the Council as regards maximum residue levels for 2,4-D, beflubutamid, cyclanilide, diniconazole, florasulam, metolachlor and S-metolachlor, and milbemectin in or on certain products. Available online: http://down.foodmate.net/standard/sort/44/39676.html (accessed on 27 March 2019).
- National Standards of People’s Republic of China. GB 2763-2016: National food safety standard- Maximum residue limits for pesticides in food. Available online: http://down.foodmate.net/standard/sort/3/50617.html (accessed on 27 March 2019).
- Wu, R.A.; Zou, H.F.; Fu, H.J. Separation of peptides on mixed mode of reversed-phase and ion-exchange capillary electrochromatography with a monolithic column. Electrophoresis 2002, 23, 1239–1245. [Google Scholar] [CrossRef] [Green Version]
- Wu, F.; Wang, J.; Zhao, Q.; Jiang, N.; Lin, X.; Xie, Z.; Li, J.; Zhang, Q. Detection of trans-fatty acids by high performance liquid chromatography coupled with in-tube solid-phase microextraction using hydrophobic polymeric monolith. J. Chromatogr. B. 2017, 1040, 214–221. [Google Scholar] [CrossRef]
Sample Availability: Not available. |






| Analytes | Linearity 1 | R2 | Linear Range (μg/kg) | LOD (μg/kg) | FE |
|---|---|---|---|---|---|
| 2,2-CPPA | Y = 533.0x + 1060.7 | 0.9957 | 5–600 | 1.5 | 85.7 |
| 2,3-CPPA | Y = 507.4x + 1017.7 | 0.9977 | 5–600 | 2.1 | 83.6 |
| 2,4-D | Y = 554.6x + 513.4 | 0.9973 | 5–600 | 1.2 | 96.7 |
| 2,4-DP | Y = 592.2x + 563.4 | 0.9996 | 5–600 | 0.9 | 98.3 |
| Analyte | Spiked/(μg/kg) | Found/(μg/kg) | Recovery 1/% | RSD/% (n = 3) |
|---|---|---|---|---|
| 2,2-CPPA | 25 | 21.8 | 87.3 | 5.2 |
| 150 | 142.3 | 94.9 | 2.7 | |
| 250 | 254.0 | 101.6 | 1.2 | |
| 2,3-CPPA | 25 | 24.6 | 98.4 | 4.7 |
| 150 | 134.0 | 89.3 | 2.3 | |
| 250 | 259.0 | 103.6 | 0.6 | |
| 2,4-D | 25 | 27.9 | 111.6 | 6.9 |
| 150 | 151.2 | 100.8 | 3.7 | |
| 250 | 232.3 | 92.9 | 1.8 | |
| 2,4-DP | 25 | 26.4 | 105.6 | 7.3 |
| 150 | 145.4 | 96.9 | 5.5 | |
| 250 | 244.3 | 97.7 | 3.4 |
| Analytes | Run-to-Run Reproducibility (n = 5) | Batch-to-Batch Reproducibility (n = 3) |
|---|---|---|
| 2,2-CPPA | 1.3 | 7.9 |
| 2,3-CPPA | 0.6 | 6.9 |
| 2,4-D | 1.9 | 5.1 |
| 2,4-DP | 2.5 | 9.3 |
| Methods | Retention Time (min) | LOD (μg/kg) 1 | ||||||
|---|---|---|---|---|---|---|---|---|
| 2,2-CPPA | 2,3-CPPA | 2,4-D | 2,4-DP | 2,2-CPPA | 2,3-CPPA | 2,4-D | 2,4-DP | |
| This method | 8.2 | 8.7 | 10.1 | 14.3 | 1.2 | 0.9 | 2.1 | 1.5 |
| LC-MS | 24.8 | 26.1 | 26.2 | 27.0 | - | - | 3.0 | 1.0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, W.; Wu, F.; Dai, Y.; Zhang, J.; Ni, B.; Wang, J. Poly (Octadecyl Methacrylate-Co-Trimethylolpropane Trimethacrylate) Monolithic Column for Hydrophobic in-Tube Solid-Phase Microextraction of Chlorophenoxy Acid Herbicides. Molecules 2019, 24, 1678. https://doi.org/10.3390/molecules24091678
Li W, Wu F, Dai Y, Zhang J, Ni B, Wang J. Poly (Octadecyl Methacrylate-Co-Trimethylolpropane Trimethacrylate) Monolithic Column for Hydrophobic in-Tube Solid-Phase Microextraction of Chlorophenoxy Acid Herbicides. Molecules. 2019; 24(9):1678. https://doi.org/10.3390/molecules24091678
Chicago/Turabian StyleLi, Wenbang, Fangling Wu, Yongwei Dai, Jing Zhang, Bichen Ni, and Jiabin Wang. 2019. "Poly (Octadecyl Methacrylate-Co-Trimethylolpropane Trimethacrylate) Monolithic Column for Hydrophobic in-Tube Solid-Phase Microextraction of Chlorophenoxy Acid Herbicides" Molecules 24, no. 9: 1678. https://doi.org/10.3390/molecules24091678