The Anti-Inflammatory Role of Mannich Curcuminoids; Special Focus on Colitis
Abstract
:1. Introduction
2. Results
2.1. Relation of Mannich Substrates to Naturally Occurring Curcuminoids
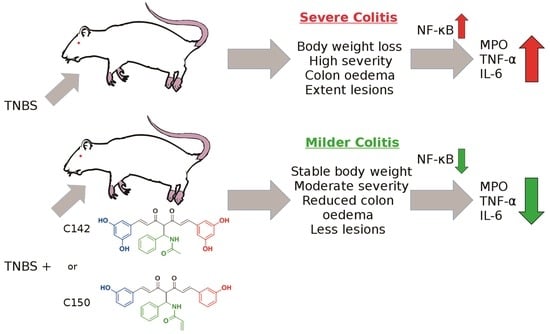
2.2. Anti-Inflammatory Effects of Curcumin Analogues in 2,4,6-trinitrobenzenesulphonic acid (TNBS) Induced Colitis
2.3. Effect of Mannich Curcuminoids on Inflammatory Mediators
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Experimental Design
4.3. Dosage and Treatment
4.4. Haematoxylin and Eosin Staining
4.5. Damage Score and Lesion Measurement
4.6. Measurement of Myeloperoxidase Activity
4.7. NF-κB Inhibition Assay
4.8. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
4.9. Data Representation and Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
References
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2018, 9, 7204–7218. [Google Scholar] [CrossRef] [PubMed]
- Hunter, P. The inflammation theory of disease. The growing realization that chronic inflammation is crucial in many diseases opens new avenues for treatment. EMBO Rep. 2012, 13, 968–970. [Google Scholar] [CrossRef] [PubMed]
- Szebeni, G.J.; Vizler, C.; Kitajka, K.; Puskas, L.G. Inflammation and cancer: Extra- and intracellular determinants of tumor-associated macrophages as tumor promoters. Med. Inflamm. 2017, 2017, 9294018. [Google Scholar] [CrossRef] [PubMed]
- Szebeni, G.J.; Vizler, C.; Nagy, L.I.; Kitajka, K.; Puskas, L.G. Pro-tumoral inflammatory myeloid cells as emerging therapeutic targets. Int. J. Mol. Sci. 2016, 17, 1958. [Google Scholar] [CrossRef]
- Bernstein, C.N.; Fried, M.; Krabshuis, J.H.; Cohen, H.; Eliakim, R.; Fedail, S.; Gearry, R.; Goh, K.L.; Hamid, S.; Khan, A.G.; et al. World gastroenterology organization practice guidelines for the diagnosis and management of ibd in 2010. Inflamm. Bowel. Dis. 2010, 16, 112–124. [Google Scholar] [CrossRef]
- Molodecky, N.A.; Soon, I.S.; Rabi, D.M.; Ghali, W.A.; Ferris, M.; Chernoff, G.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Barkema, H.W.; et al. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology 2012, 142, 46–54. [Google Scholar] [CrossRef]
- Szalai, Z.; Szasz, A.; Nagy, I.; Puskas, L.G.; Kupai, K.; Kiraly, A.; Berko, A.M.; Posa, A.; Strifler, G.; Barath, Z.; et al. Anti-inflammatory effect of recreational exercise in tnbs-induced colitis in rats: Role of nos/ho/mpo system. Oxid. Med. Cell Longev. 2014, 2014, 925981. [Google Scholar] [CrossRef]
- Talapka, P.; Nagy, L.I.; Pal, A.; Poles, M.Z.; Berko, A.; Bagyanszki, M.; Puskas, L.G.; Fekete, E.; Bodi, N. Alleviated mucosal and neuronal damage in a rat model of crohn’s disease. World J. Gastroenterol. 2014, 20, 16690–16697. [Google Scholar] [CrossRef]
- Talapka, P.; Berko, A.; Nagy, L.I.; Chandrakumar, L.; Bagyanszki, M.; Puskas, L.G.; Fekete, E.; Bodi, N. Structural and molecular features of intestinal strictures in rats with crohn’s-like disease. World J. Gastroenterol. 2016, 22, 5154–5164. [Google Scholar] [CrossRef]
- Ford, A.C.; Moayyedi, P.; Hanauer, S.B. Ulcerative colitis. BMJ 2013, 346, f432. [Google Scholar] [CrossRef]
- Meier, J.; Sturm, A. Current treatment of ulcerative colitis. World J. Gastroenterol. 2011, 17, 3204–3212. [Google Scholar] [PubMed]
- Ordas, I.; Eckmann, L.; Talamini, M.; Baumgart, D.C.; Sandborn, W.J. Ulcerative colitis. Lancet 2012, 380, 1606–1619. [Google Scholar] [CrossRef] [Green Version]
- Basnet, P.; Skalko-Basnet, N. Curcumin: An anti-inflammatory molecule from a curry spice on the path to cancer treatment. Molecules 2011, 16, 4567–4598. [Google Scholar] [CrossRef] [PubMed]
- Hewlings, S.J.; Kalman, D.S. Curcumin: A review of its’ effects on human health. Foods 2017, 6, 92. [Google Scholar] [CrossRef] [PubMed]
- Szebeni, G.J.; Balazs, A.; Madarasz, I.; Pocz, G.; Ayaydin, F.; Kanizsai, I.; Fajka-Boja, R.; Alfoldi, R.; Hackler, L., Jr.; Puskas, L.G. Achiral mannich-base curcumin analogs induce unfolded protein response and mitochondrial membrane depolarization in panc-1 cells. Int. J. Mol. Sci. 2017, 18, 2105. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.L.; Hsu, C.H.; Lin, J.K.; Hsu, M.M.; Ho, Y.F.; Shen, T.S.; Ko, J.Y.; Lin, J.T.; Lin, B.R.; Ming-Shiang, W.; et al. Phase i clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer Res. 2001, 21, 2895–2900. [Google Scholar]
- Lao, C.D.; Ruffin, M.T.t.; Normolle, D.; Heath, D.D.; Murray, S.I.; Bailey, J.M.; Boggs, M.E.; Crowell, J.; Rock, C.L.; Brenner, D.E. Dose escalation of a curcuminoid formulation. BMC Complement. Altern. Med. 2006, 6, 10. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.C.; Patchva, S.; Aggarwal, B.B. Therapeutic roles of curcumin: Lessons learned from clinical trials. AAPS J. 2013, 15, 195–218. [Google Scholar] [CrossRef]
- Liu, W.; Zhai, Y.; Heng, X.; Che, F.Y.; Chen, W.; Sun, D.; Zhai, G. Oral bioavailability of curcumin: Problems and advancements. J. Drug Target 2016, 24, 694–702. [Google Scholar] [CrossRef]
- Gyuris, M.; Hackler, L., Jr.; Nagy, L.I.; Alfoldi, R.; Redei, E.; Marton, A.; Vellai, T.; Farago, N.; Ozsvari, B.; Hetenyi, A.; et al. Mannich curcuminoids as potent anticancer agents. Arch. Pharm. (Weinheim) 2017, 350, e1700005. [Google Scholar] [CrossRef]
- Nagy, L.I.; Feher, L.Z.; Szebeni, G.J.; Gyuris, M.; Sipos, P.; Alfoldi, R.; Ozsvari, B.; Hackler, L., Jr.; Balazs, A.; Batar, P.; et al. Curcumin and its analogue induce apoptosis in leukemia cells and have additive effects with bortezomib in cellular and xenograft models. Biomed. Res. Int. 2015, 2015, 968981. [Google Scholar] [CrossRef] [PubMed]
- Hackler, L., Jr.; Ozsvari, B.; Gyuris, M.; Sipos, P.; Fabian, G.; Molnar, E.; Marton, A.; Farago, N.; Mihaly, J.; Nagy, L.I. The curcumin analog c-150, influencing nf-kappab, upr and akt/notch pathways has potent anticancer activity in vitro and in vivo. PLoS ONE 2016, 11, e0149832. [Google Scholar] [CrossRef] [PubMed]
- Mao, H.; Wan, J.; Pan, Y. Facile and diastereoselective synthesis of β-acetamido ketones and keto esters via direct mannich-type reaction. Tetrahedron 2009, 65, 1026–1032. [Google Scholar] [CrossRef]
- Horvath, K.; Varga, C.; Berko, A.; Posa, A.; Laszlo, F.; Whittle, B.J. The involvement of heme oxygenase-1 activity in the therapeutic actions of 5-aminosalicylic acid in rat colitis. Eur. J. Pharmacol. 2008, 581, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Boughton-Smith, N.K.; Wallace, J.L.; Whittle, B.J. Relationship between arachidonic acid metabolism, myeloperoxidase activity and leukocyte infiltration in a rat model of inflammatory bowel disease. Agents Act. 1988, 25, 115–123. [Google Scholar] [CrossRef]
- Khan, A.A.; Alsahli, M.A.; Rahmani, A.H. Myeloperoxidase as an active disease biomarker: Recent biochemical and pathological perspectives. Med. Sci. 2018, 6, 33. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Lenardo, M.J.; Baltimore, D. 30 years of nf-kappa b: A blossoming of relevance to human pathobiology. Cell 2017, 168, 37–57. [Google Scholar] [CrossRef] [PubMed]
- Vlahopoulos, S.A. Aberrant control of nf-kappab in cancer permits transcriptional and phenotypic plasticity, to curtail dependence on host tissue: Molecular mode. Cancer Biol. Med. 2017, 14, 254–270. [Google Scholar] [PubMed]
- Andresen, L.; Jorgensen, V.L.; Perner, A.; Hansen, A.; Eugen-Olsen, J.; Rask-Madsen, J. Activation of nuclear factor kappab in colonic mucosa from patients with collagenous and ulcerative colitis. Gut 2005, 54, 503–509. [Google Scholar] [CrossRef]
- Atreya, I.; Atreya, R.; Neurath, M.F. Nf-kappab in inflammatory bowel disease. J. Intern. Med. 2008, 263, 591–596. [Google Scholar] [CrossRef]
- Wang, X.Y.; Quinn, P.J. Endotoxins: Lipopolysaccharides of gram-negative bacteria. Endotoxins 2010, 53, 3–25. [Google Scholar]
- Deree, J.; Martins, J.O.; Melbostad, H.; Loomis, W.H.; Coimbra, R. Insights into the regulation of tnf-alpha production in human mononuclear cells: The effects of non-specific phosphodiesterase inhibition. Clinics 2008, 63, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Libermann, T.A.; Baltimore, D. Activation of interleukin-6 gene expression through the nf-kappa b transcription factor. Mol. Cell. Biol. 1990, 10, 2327–2334. [Google Scholar] [CrossRef] [PubMed]
- Levin, A.D.; Wildenberg, M.E.; van den Brink, G.R. Mechanism of action of anti-tnf therapy in inflammatory bowel disease. J. Crohns. Colitis 2016, 10, 989–997. [Google Scholar] [CrossRef] [PubMed]
- Parisinos, C.A.; Serghiou, S.; Katsoulis, M.; George, M.J.; Patel, R.S.; Hemingway, H.; Hingorani, A.D. Variation in interleukin 6 receptor gene associates with risk of crohn’s disease and ulcerative colitis. Gastroenterology 2018, 155, 303–306 e302. [Google Scholar] [CrossRef]
- Mukherjee, S.; Chen, L.Y.; Papadimos, T.J.; Huang, S.; Zuraw, B.L.; Pan, Z.K. Lipopolysaccharide-driven th2 cytokine production in macrophages is regulated by both myd88 and tram. J. Biol. Chem. 2009, 284, 29391–29398. [Google Scholar] [CrossRef] [PubMed]
- Van Kampen, C.; Gauldie, J.; Collins, S.M. Proinflammatory properties of il-4 in the intestinal microenvironment. Am. J. Physiol. Gastrointest. Liver Physiol. 2005, 288, G111–G117. [Google Scholar] [CrossRef]
- Toden, S.; Theiss, A.L.; Wang, X.; Goel, A. Essential turmeric oils enhance anti-inflammatory efficacy of curcumin in dextran sulfate sodium-induced colitis. Sci. Rep. 2017, 7, 814. [Google Scholar] [CrossRef] [PubMed]
- Baliga, M.S.; Joseph, N.; Venkataranganna, M.V.; Saxena, A.; Ponemone, V.; Fayad, R. Curcumin, an active component of turmeric in the prevention and treatment of ulcerative colitis: Preclinical and clinical observations. Food Funct. 2012, 3, 1109–1117. [Google Scholar] [CrossRef]
- Jian, Y.T.; Mai, G.F.; Wang, J.D.; Zhang, Y.L.; Luo, R.C.; Fang, Y.X. Preventive and therapeutic effects of nf-kappab inhibitor curcumin in rats colitis induced by trinitrobenzene sulfonic acid. World J. Gastroenterol. 2005, 11, 1747–1752. [Google Scholar] [CrossRef]
- Wang, Y.J.; Pan, M.H.; Cheng, A.L.; Lin, L.I.; Ho, Y.S.; Hsieh, C.Y.; Lin, J.K. Stability of curcumin in buffer solutions and characterization of its degradation products. J. Pharm. Biomed. Anal. 1997, 15, 1867–1876. [Google Scholar] [CrossRef]
- Mondal, S.; Ghosh, S.; Moulik, S.P. Stability of curcumin in different solvent and solution media: Uv-visible and steady-state fluorescence spectral study. J. Photochem. Photobiol. B 2016, 158, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Rego-Filho, F.G.; de Araujo, M.T.; de Oliveira, K.T.; Bagnato, V.S. Validation of photodynamic action via photobleaching of a new curcumin-based composite with enhanced water solubility. J. Fluoresc. 2014, 24, 1407–1413. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wu, J.; Wang, J.K. A database and functional annotation of nf-kappa b target genes. Int. J. Clin. Exp. Med. 2016, 9, 7986–7995. [Google Scholar]
- Morris, G.P.; Beck, P.L.; Herridge, M.S.; Depew, W.T.; Szewczuk, M.R.; Wallace, J.L. Hapten-induced model of chronic inflammation and ulceration in the rat colon. Gastroenterology 1989, 96, 795–803. [Google Scholar] [CrossRef]
- Bradley, P.P.; Priebat, D.A.; Christensen, R.D.; Rothstein, G. Measurement of cutaneous inflammation: Estimation of neutrophil content with an enzyme marker. J. Investig. Dermatol. 1982, 78, 206–209. [Google Scholar] [CrossRef]
- Szebeni, G.J.; Balog, J.A.; Demjen, A.; Alfoldi, R.; Vegi, V.L.; Feher, L.Z.; Man, I.; Kotogany, E.; Guban, B.; Batar, P.; et al. Imidazo[1,2-b]pyrazole-7-carboxamides induce apoptosis in human leukemia cells at nanomolar concentrations. Molecules 2018, 23, 2845. [Google Scholar] [CrossRef]
- Szebeni, G.J.; Tancos, Z.; Feher, L.Z.; Alfoldi, R.; Kobolak, J.; Dinnyes, A.; Puskas, L.G. Real architecture for 3d tissue (raft) culture system improves viability and maintains insulin and glucagon production of mouse pancreatic islet cells. Cytotechnology 2017, 69, 359–369. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available via contacting the corresponding author |








© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szebeni, G.J.; Nagy, L.I.; Berkó, A.; Hoffmann, A.; Fehér, L.Z.; Bagyánszki, M.; Kari, B.; Balog, J.A.; Hackler, L., Jr.; Kanizsai, I.; et al. The Anti-Inflammatory Role of Mannich Curcuminoids; Special Focus on Colitis. Molecules 2019, 24, 1546. https://doi.org/10.3390/molecules24081546
Szebeni GJ, Nagy LI, Berkó A, Hoffmann A, Fehér LZ, Bagyánszki M, Kari B, Balog JA, Hackler L Jr., Kanizsai I, et al. The Anti-Inflammatory Role of Mannich Curcuminoids; Special Focus on Colitis. Molecules. 2019; 24(8):1546. https://doi.org/10.3390/molecules24081546
Chicago/Turabian StyleSzebeni, Gábor J., Lajos I. Nagy, Anikó Berkó, Alexandra Hoffmann, Liliána Z. Fehér, Mária Bagyánszki, Beáta Kari, József A. Balog, László Hackler, Jr., Iván Kanizsai, and et al. 2019. "The Anti-Inflammatory Role of Mannich Curcuminoids; Special Focus on Colitis" Molecules 24, no. 8: 1546. https://doi.org/10.3390/molecules24081546