Antioxidant Activities of Quercetin and Its Complexes for Medicinal Application
Abstract
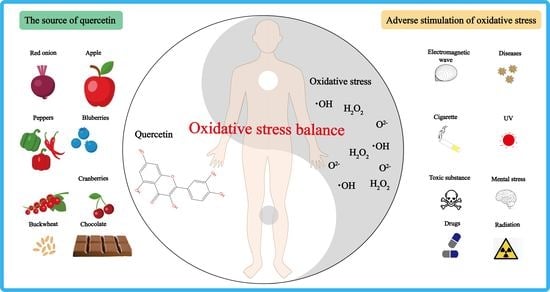
:1. Introduction
2. Antioxidant Activity of Quercetin In Vivo
2.1. Direct Effects of Quercetin on GSH
2.2. Effects of Quercetin on Enzymatic Activity
2.3. Effects of Quercetin On Signal Transduction Pathways
2.4. Effects of Quercetin on ROS Caused by Environmental and Toxicological Factors
3. Chemical Studies on the Antioxidant Activity of Quercetin
3.1. Complexes with Metal Ions
3.2. Complexes with Complex Ions
4. Application of Antioxidant Activity in the Medicinal Field
4.1. Effects of Quercetin on Tumors
4.2. Effects of Quercetin on Heart Diseases
4.3. Effects of Quercetin on Depression
4.4. Effects of Quercetin on Diseases Caused by Poisoning
4.5. Effects of Quercetin on Other Diseases
5. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Rauf, A.; Imran, M.; Khan, I.A.; Ur-Rehman, M.; Gilani, S.A.; Mehmood, Z.; Mubarak, M.S. Anticancer potential of quercetin: A comprehensive review. Phytother. Res. 2018, 32, 2109–2130. [Google Scholar] [CrossRef] [PubMed]
- Almeida, A.F.; Borge, G.I.A.; Piskula, M.; Tudose, A.; Tudoreanu, L.; Valentova, K.; Williamson, G.; Santos, C.N. Bioavailability of Quercetin in Humans with a Focus on Interindividual Variation. Compr. Rev. Food Sci. Food Saf. 2018, 17, 714–731. [Google Scholar] [CrossRef] [Green Version]
- Anand David, A.V.; Arulmoli, R.; Parasuraman, S. Overviews of Biological Importance of Quercetin: A Bioactive Flavonoid. Pharmacogn. Rev. 2016, 10, 84–89. [Google Scholar] [PubMed] [Green Version]
- Boots, A.W.; Haenen, G.; Bast, A. Health effects of quercetin: From antioxidant to nutraceutical. Eur. J. Pharmacol. 2008, 585, 325–337. [Google Scholar] [CrossRef]
- Xie, X.; Shen, Q.; Cao, L.; Chen, Y.; Ma, L.; Xiao, Q.; Yu, C.; Fu, Z. Depression caused by long-term stress regulates premature aging and is possibly associated with disruption of circadian rhythms in mice. Physiol. Behav. 2019, 199, 100–110. [Google Scholar] [CrossRef]
- Halevas, E. Encapsulation of flavonoid quercetin in PEGylated SiO2 nanoparticles against Cu (II)-induced oxidative stress. Hell. J. Nucl. Med. 2017, 20, 156–168. [Google Scholar]
- Aytac, Z.; Kusku, S.I.; Durgun, E.; Uyar, T. Quercetin/β-cyclodextrin inclusion complex embedded nanofibres: Slow release and high solubility. Food Chem. 2016, 197, 864–871. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anandam, S.; Selvamuthukumar, S. Fabrication of cyclodextrin nanosponges for quercetin delivery: Physicochemical characterization, photostability, and antioxidant effects. J. Mater. Sci. 2014, 49, 8140–8153. [Google Scholar] [CrossRef]
- Chen, C. CiteSpace II: Detecting and visualizing emerging trends and transient patterns in scientific literature. J. Am. Soc. Inf. Sci. Technol. 2006, 57, 359–377. [Google Scholar] [CrossRef] [Green Version]
- Liao, H.; Tang, M.; Luo, L.; Li, C.; Chiclana, F.; Zeng, X.J. A bibliometric analysis and visualization of medical big data research. Sustainability 2018, 10, 166. [Google Scholar] [CrossRef]
- Tejada, S.; Nabavi, S.M.; Capo, X.; Martorell, M.; Bibiloni, M.D.; Tur, J.A.; Pons, A.; Sureda, A. Quercetin Effects on Exercise Induced Oxidative Stress and Inflammation. Curr. Org. Chem. 2017, 21, 348–356. [Google Scholar] [CrossRef]
- Lesjak, M.; Beara, I.; Simin, N.; Pintac, D.; Majkic, T.; Bekvalac, K.; Orcic, D.; Mimica-Dukic, N. Antioxidant and anti-inflammatory activities of quercetin and its derivatives. J. Funct. Foods 2018, 40, 68–75. [Google Scholar] [CrossRef]
- Kobori, M.; Takahashi, Y.; Akimoto, Y.; Sakurai, M.; Matsunaga, I.; Nishimuro, H.; Ippoushi, K.; Oike, H.; Ohnishi-Kameyama, M. Chronic high intake of quercetin reduces oxidative stress and induces expression of the antioxidant enzymes in the liver and visceral adipose tissues in mice. J. Funct. Foods 2015, 15, 551–560. [Google Scholar] [CrossRef]
- Belen Granado-Serrano, A.; Angeles Martin, M.; Bravo, L.; Goya, L.; Ramos, S. Quercetin modulates Nrf2 and glutathione-related defenses in HepG2 cells: Involvement of p38. Chem. Biol. Interact. 2012, 195, 154–164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kinaci, M.K.; Erkasap, N.; Kucuk, A.; Koken, T.; Tosun, M. Effects of quercetin on apoptosis, NF-κB and NOS gene expression in renal ischemia/reperfusion injury. Exp. Ther. Med. 2012, 3, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.N.; Pu, L.L.; Chen, M.; Wei, J.Y.; Xin, Z.H.; Wang, Y.W.; Yao, Z.X.; Shi, T.L.; Guo, C. Glutathione homeostasis is significantly altered by quercetin via the Keapl/Nrf2 and MAPK signaling pathways in rats. J. Clin. Biochem. Nutr. 2018, 62, 56–62. [Google Scholar] [CrossRef]
- Ademosun, A.O.; Oboh, G.; Bello, F.; Ayeni, P.O. Antioxidative Properties and Effect of Quercetin and Its Glycosylated Form (Rutin) on Acetylcholinesterase and Butyrylcholinesterase Activities. J. Evid. Based Complement. Altern. Med. 2016, 21, Np11–Np17. [Google Scholar] [CrossRef] [PubMed]
- Adedara, I.A.; Ego, V.C.; Subair, T.I.; Oyediran, O.; Farombi, E.O. Quercetin Improves Neurobehavioral Performance through Restoration of Brain Antioxidant Status and Acetylcholinesterase Activity in Manganese-Treated Rats. Neurochem. Res. 2017, 42, 1219–1229. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.H.; Park, J.H.; Ahn, J.H.; Cho, J.H.; Kim, I.H.; Lee, J.C.; Won, M.H.; Lee, C.H.; Hwang, I.K.; Kim, J.D.; et al. Pretreated quercetin protects gerbil hippocampal CA1 pyramidal neurons from transient cerebral ischemic injury by increasing the expression of antioxidant enzymes. Neural Regen. Res. 2017, 12, 220–227. [Google Scholar] [PubMed]
- Braun, K.F.; Ehnert, S.; Freude, T.; Egana, J.T.; Schenck, T.L.; Buchholz, A.; Schmitt, A.; Siebenlist, S.; Schyschka, L.; Neumaier, M.; et al. Quercetin protects primary human osteoblasts exposed to cigarette smoke through activation of the antioxidative enzymes HO-1 and SOD-1. Sci. World J. 2011, 11, 2348–2357. [Google Scholar] [CrossRef]
- Akkoyun, D.C.; Akyuz, A.; Dogan, M.; Erboga, M.; Aktas, C.; Caglar, V.; Uygur, R.; Topcu, B.; Yilmaz, A.; Gurel, A. Quercetin Inhibits Heart Injury in Lipopolysaccharide-induced Endotoxemic Model by Suppressing the Effects of Reactive Oxygen Species. Anal. Quant. Cytopathol. Histopathol. 2016, 38, 183–188. [Google Scholar]
- Kawamura, K.; Qi, F.; Kobayashi, J. Potential relationship between the biological effects of low-dose irradiation and mitochondrial ROS production. J. Radiat. Res. 2018, 59, ii91–ii97. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Hu, X.; Liu, Y.; Dong, S.; Wen, Z.; He, W.; Zhang, S.; Huang, Q.; Shi, M. ROS signaling under metabolic stress: Cross-talk between AMPK and AKT pathway. Mol. Cancer 2017, 16, 79. [Google Scholar] [CrossRef] [PubMed]
- Jalmi, S.K.; Sinha, A.K. ROS mediated MAPK signaling in abiotic and biotic stress-striking similarities and differences. Front. Plant Sci. 2015, 6, 769. [Google Scholar] [CrossRef]
- Stoiber, W.; Obermayer, A.; Steinbacher, P.; Krautgartner, W.D. The role of reactive oxygen species (ROS) in the formation of extracellular traps (ETs) in humans. Biomolecules 2015, 5, 702–723. [Google Scholar] [CrossRef] [PubMed]
- Vurusaner, B.; Poli, G.; Basaga, H. Tumor suppressor genes and ROS: Complex networks of interactions. Free Radic. Biol. Med. 2012, 52, 7–18. [Google Scholar] [CrossRef]
- Song, Y.; Liu, J.; Zhang, F.; Zhang, J.; Shi, T.; Zeng, Z. Antioxidant effect of quercetin against acute spinal cord injury in rats and its correlation with the p38MAPK/iNOS signaling pathway. Life Sci. 2013, 92, 1215–1221. [Google Scholar] [CrossRef]
- Chen, H.; Lu, C.; Liu, H.; Wang, M.; Zhao, H.; Yan, Y.; Han, L. Quercetin ameliorates imiquimod-induced psoriasis-like skin inflammation in mice via the NF-κB pathway. Int. Immunopharmacol. 2017, 48, 110–117. [Google Scholar] [CrossRef]
- Hung, C.H.; Chan, S.H.; Chu, P.M.; Tsai, K.L. Quercetin is a potent anti-atherosclerotic compound by activation of SIRT1 signaling under oxLDL stimulation. Mol. Nutr. Food Res. 2015, 59, 1905–1917. [Google Scholar] [CrossRef]
- Lu, X.L.; Zhao, C.H.; Yao, X.L.; Zhang, H. Quercetin attenuates high fructose feeding-induced atherosclerosis by suppressing inflammation and apoptosis via ROS-regulated PI3K/AKT signaling pathway. Biomed. Pharmacother. 2017, 85, 658–671. [Google Scholar] [CrossRef]
- Chang, H.C.; Yang, Y.R.; Wang, P.S.; Wang, R.Y. Quercetin enhances exercise-mediated neuroprotective effects in brain ischemic rats. Med. Sci. Sports Exerc. 2014, 46, 1908–1916. [Google Scholar] [CrossRef]
- Du, L.; Hao, M.; Li, C.; Wu, W.; Wang, W.; Ma, Z.; Yang, T.; Zhang, N.; Isaac, A.T.; Zhu, X.; et al. Quercetin inhibited epithelial mesenchymal transition in diabetic rats, high-glucose-cultured lens, and SRA01/04 cells through transforming growth factor-beta2/phosphoinositide 3-kinase/Akt pathway. Mol. Cell. Endocrinol. 2017, 452, 44–56. [Google Scholar] [CrossRef]
- Peng, Z.; Gong, X.; Yang, Y.; Huang, L.; Zhang, Q.; Zhang, P.; Wan, R.; Zhang, B. Hepatoprotective effect of quercetin against LPS/d-GalN induced acute liver injury in mice by inhibiting the IKK/NF-κB and MAPK signal pathways. Int. Immunopharmacol. 2017, 52, 281–289. [Google Scholar] [CrossRef]
- Jin, Y.; Huang, Z.L.; Li, L.; Yang, Y.; Wang, C.H.; Wang, Z.T.; Ji, L.L. Quercetin attenuates toosendanin-induced hepatotoxicity through inducing the Nrf2/GCL/GSH antioxidant signaling pathway. Acta Pharmacol. Sin. 2019, 40, 75. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Zhou, X.; Zhao, J. Quercetin prevents alcohol-induced liver injury through targeting of PI3K/Akt/nuclear factor-κB and STAT3 signaling pathway. Exp. Ther. Med. 2017, 14, 6169–6175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, L.L.; Sheng, Y.C.; Zheng, Z.Y.; Shi, L.; Wang, Z.T. The involvement of p62-Keap1-Nrf2 antioxidative signaling pathway and JNK in the protection of natural flavonoid quercetin against hepatotoxicity. Free Radic. Biol. Med. 2015, 85, 12–23. [Google Scholar] [CrossRef]
- Dong, Q.H.; Chen, L.; Lu, Q.W.; Sharma, S.; Li, L.; Morimoto, S.; Wang, G.Y. Quercetin attenuates doxorubicin cardiotoxicity by modulating Bmi-1 expression. Br. J. Pharmacol. 2014, 171, 4440–4454. [Google Scholar] [CrossRef] [Green Version]
- Ma, J.Q.; Li, Z.; Xie, W.R.; Liu, C.M.; Liu, S.S. Quercetin protects mouse liver against CCl(4)-induced inflammation by the TLR2/4 and MAPK/NF-κB pathway. Int. Immunopharmacol. 2015, 28, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.M.; Zheng, G.H.; Ming, Q.L.; Sun, J.M.; Cheng, C. Protective effect of quercetin on lead-induced oxidative stress and endoplasmic reticulum stress in rat liver via the IRE1/JNK and PI3K/Akt pathway. Free Radic. Res. 2013, 47, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Shukla, R.K.; Chandravanshi, L.P.; Srivastava, P.; Dhuriya, Y.K.; Shanker, J.; Singh, M.P.; Pant, A.B.; Khanna, V.K. Protective Role of Quercetin in Cadmium-Induced Cholinergic Dysfunctions in Rat Brain by Modulating Mitochondrial Integrity and MAP Kinase Signaling. Mol. Neurobiol. 2017, 54, 4560–4583. [Google Scholar] [CrossRef] [PubMed]
- Pratheeshkumar, P.; Son, Y.O.; Divya, S.P.; Wang, L.; Turcios, L.; Roy, R.V.; Hitron, J.A.; Kim, D.; Dai, J.; Asha, P.; et al. Quercetin inhibits Cr (VI)-induced malignant cell transformation by targeting miR-21-PDCD4 signaling pathway. Oncotarget 2017, 8, 52118–52131. [Google Scholar] [CrossRef] [PubMed]
- Dong, F.; Wang, S.; Wang, Y.; Yang, X.; Jiang, J.; Wu, D.; Qu, X.; Fan, H.; Yao, R. Quercetin ameliorates learning and memory via the Nrf2-ARE signaling pathway in d-galactose-induced neurotoxicity in mice. Biochem. Biophys. Res. Commun. 2017, 491, 636–641. [Google Scholar] [CrossRef]
- Wattel, A.; Kamel, S.; Prouillet, C.; Petit, J.P.; Lorget, F.; Offord, E.; Brazier, M. Flavonoid quercetin differentiation decreases osteoclastic induced by RANKL via a mechanism involving NF-κB and AP-1. J. Cell. Biochem. 2004, 92, 285–295. [Google Scholar] [CrossRef]
- Jin, X.; Su, R.; Li, R.; Song, L.; Chen, M.; Cheng, L.; Li, Z. Amelioration of particulate matter-induced oxidative damage by vitamin c and quercetin in human bronchial epithelial cells. Chemosphere 2016, 144, 459–466. [Google Scholar] [CrossRef]
- Casagrande, R.; Georgetti, S.R.; Verri, W.A.; Dorta, D.J.; dos Santos, A.C.; Fonseca, M.J.V. Protective effect of topical formulations containing quercetin against UVB-induced oxidative stress in hairless mice. J. Photochem. Photobiol. B 2006, 84, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.B.; Li, N.; Wang, Y.L.; Ding, L.; Chen, H.J.; Yu, Y.H.; Shi, X.J. Protective effects of quercetin on UVB irradiation-induced cytotoxicity through ROS clearance in keratinocyte cells. Oncol. Rep. 2017, 37, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Zerin, T.; Kim, Y.S.; Hong, S.Y.; Song, H.Y. Quercetin reduces oxidative damage induced by paraquat via modulating expression of antioxidant genes in A549 cells. J. Appl. Toxicol. 2013, 33, 1460–1467. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.T.; Ding, C.; Zhou, N.; Xu, C. Quercetin protects gastric epithelial cell from oxidative damage in vitro and in vivo. Eur. J. Pharmacol. 2015, 754, 115–124. [Google Scholar] [CrossRef]
- Tvrda, E.; Tusimova, E.; Kovacik, A.; Paal, D.; Libova, L.; Lukac, N. Protective Effects of Quercetin on Selected Oxidative Biomarkers in Bovine Spermatozoa Subjected to Ferrous Ascorbate. Reprod. Domest. Anim. 2016, 51, 524–537. [Google Scholar] [CrossRef]
- Kale, A.; Pişkin, Ö.; Baş, Y.; Aydın, B.G.; Can, M.; Elmas, Ö.; Büyükuysal, Ç. Neuroprotective effects of Quercetin on radiation-induced brain injury in rats. J. Radiat. Res. 2018, 59, 404–410. [Google Scholar] [CrossRef] [Green Version]
- Zargar, S.; Siddiqi, N.J.; Ansar, S.; Alsulaimani, M.S.; El Ansary, A.K. Therapeutic role of quercetin on oxidative damage induced by acrylamide in rat brain. Pharm. Biol. 2016, 54, 1763–1767. [Google Scholar] [CrossRef] [PubMed]
- Ola, M.S.; Ahmed, M.M.; Shams, S.; Al-Rejaie, S.S. Neuroprotective effects of quercetin in diabetic rat retina. Saudi J. Biol. Sci. 2017, 24, 1186–1194. [Google Scholar] [CrossRef] [PubMed]
- Heo, H.J.; Lee, C.Y. Protective effects of quercetin and vitamin C against oxidative stress-induced neurodegeneration. J. Agric. Food Chem. 2004, 52, 7514–7517. [Google Scholar] [CrossRef] [PubMed]
- Zargar, S.; Siddiqi, N.J.; Al Daihan, S.K.; Wani, T.A. Protective effects of quercetin on cadmium fluoride induced oxidative stress at different intervals of time in mouse liver. Acta Biochim. Pol. 2015, 62, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.L.; Rao, N.B.; Somashekarappa, H.M.; Rajashekhar, K.P. Antigenotoxic potential of rutin and quercetin in Swiss mice exposed to gamma radiation. Biomed. J. 2014, 37, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Ozyurt, H.; Cevik, O.; Ozgen, Z.; Ozden, A.S.; Cadirci, S.; Elmas, M.A.; Ercan, F.; Goren, M.Z.; Sener, G. Quercetin protects radiation-induced DNA damage and apoptosis in kidney and bladder tissues of rats. Free Radic. Res. 2014, 48, 1247–1255. [Google Scholar] [CrossRef]
- Patil, S.L.; Mallaiah, S.H.; Patil, R.K. Antioxidative and radioprotective potential of rutin and quercetin in Swiss albino mice exposed to gamma radiation. J. Med. Phys. 2013, 38, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Kalantari, H.; Foruozandeh, H.; Khodayar, M.J.; Siahpoosh, A.; Saki, N.; Kheradmand, P. Antioxidant and hepatoprotective effects of Capparis spinosa L. fractions and Quercetin on tert-butyl hydroperoxide- induced acute liver damage in mice. J. Tradit. Complement. Med. 2018, 81, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Prasad, J.; Baitharu, I.; Sharma, A.K.; Dutta, R.; Prasad, D.; Singh, S.B. Quercetin reverses hypobaric hypoxia-induced hippocampal neurodegeneration and improves memory function in the rat. High Alt. Med. Biol. 2013, 14, 383–394. [Google Scholar] [CrossRef]
- Yarahmadi, A.; Zal, F.; Bolouki, A. Protective effects of quercetin on nicotine induced oxidative stress in ‘HepG2 cells’. Toxicol. Mech. Methods 2017, 27, 609–614. [Google Scholar] [CrossRef]
- Chen, X.; Yin, O.Q.P.; Zuo, Z.; Chow, M.S.S. Pharmacokinetics and modeling of quercetin and metabolites. Pharm. Res. 2005, 22, 892–901. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; Banerjee, S.; Chakraborty, T. Vanadium quercetin complex attenuates mammary cancer by regulating the P53, Akt/mTOR pathway and downregulates cellular proliferation correlated with increased apoptotic events. Biometals 2018, 31, 647–671. [Google Scholar] [CrossRef] [PubMed]
- Bratu, M.; Birghila, S.; Miresan, H.; Negreanu-Pirjol, T.; Prajitura, C.; Calinescu, M. Biological Activities of Zn (II) and Cu (II) Complexes with Quercetin and Rutin: Antioxidant Properties and UV-Protection Capacity. Rev. Chim. 2014, 65, 544–549. [Google Scholar]
- Bukhari, S.B.; Memon, S.; Mahroof-Tahir, M.; Bhanger, M.I. Synthesis, characterization and antioxidant activity copper-quercetin complex. Spectrochim. Acta Part A 2009, 71, 1901–1906. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, N.; Chakraborty, T.; Mallick, S.; Mana, S.; Singha, D.; Ghosh, B.; Roy, S. Synthesis, characterization and study of antioxidant activity of quercetin-magnesium complex. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 151, 807–813. [Google Scholar] [CrossRef] [PubMed]
- Raza, A.; Xu, X.; Xia, L.; Xia, C.; Tang, J.; Ouyang, Z. Quercetin-Iron Complex: Synthesis, Characterization, Antioxidant, DNA Binding, DNA Cleavage, and Antibacterial Activity Studies. J. Fluoresc. 2016, 26, 2023–2031. [Google Scholar] [CrossRef]
- Roy, S.; Das, R.; Ghosh, B.; Chakraborty, T. Deciphering the biochemical and molecular mechanism underlying the in vitro and in vivo chemotherapeutic efficacy of ruthenium quercetin complex in colon cancer. Mol. Carcinog. 2018, 57, 700–721. [Google Scholar] [CrossRef] [PubMed]
- Trifunschi, S.; Ardelean, D. Synthesis, Characterization and Antioxidant Activity of Co (II) and Cd (II) Complexes with Quercetin. Rev. Chim. 2016, 67, 2422–2424. [Google Scholar]
- Simoes, V.D.; Favarin, L.R.V.; Cabeza, N.A.; de Oliveira, T.D.; Fiorucci, A.R.; Stropa, J.M.; Rodrigues, D.C.M.; Cavalheiro, A.A.; dos Anjos, A. Synthesis, characterization and study of the properties of a new mononuclear quercetin complex containing Ga (III) ions. Quim. Nova 2013, 36, 495–501. [Google Scholar]
- Zhou, J.; Wang, L.F.; Wang, J.Y.; Tang, N. Synthesis, characterization, antioxidative and antitumor activities of solid quercetin rare earth (III) complexes. J. Inorg. Biochem. 2001, 83, 41–48. [Google Scholar] [CrossRef]
- Ravichandran, R.; Rajendran, M.; Devapiriam, D. Structural characterization and physicochemical properties of quercetin-Pb complex. J. Coord. Chem. 2014, 67, 1449–1462. [Google Scholar] [CrossRef]
- Ezzati Nazhad Dolatabadi, J.; Mokhtarzadeh, A.; Ghareghoran, S.M.; Dehghan, G. Synthesis, Characterization and Antioxidant Property of Quercetin-Tb (III) Complex. Adv. Pharm. Bull. 2014, 4, 101–104. [Google Scholar] [PubMed]
- Xu, X.R.; Yu, H.T.; Yang, Y.; Hang, L.; Yang, X.W.; Ding, S.H. Quercetin phospholipid complex significantly protects against oxidative injury in ARPE-19 cells associated with activation of Nrf2 pathway. Eur. J. Pharmacol. 2016, 770, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Rawat, M.S.; Semalty, A.; Semalty, M. Quercetin-phospholipid complex: An amorphous pharmaceutical system in herbal drug delivery. Curr. Drug Discov. Technol. 2012, 9, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Oliver, S.; Yee, E.; Kavallaris, M.; Vittorio, O.; Boyer, C. Water Soluble Antioxidant Dextran-Quercetin Conjugate with Potential Anticancer Properties. Macromol. Biosci. 2018, 18, e1700239. [Google Scholar] [CrossRef] [PubMed]
- Patra, M.; Mukherjee, R.; Banik, M.; Dutta, D.; Begum, N.A.; Basu, T. Calcium phosphate-quercetin nanocomposite (CPQN): A multi-functional nanoparticle having pH indicating, highly fluorescent and anti-oxidant properties. Colloids Surf. B. Biointerfaces 2017, 154, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.J.; Yang, F.; Zhang, L.; Pi, J.; Cai, J.Y.; Yang, P.H. Facile synthesis of multifunctional germanium nanoparticles as a carrier of quercetin to achieve enhanced biological activity. Chem. Asian J. 2014, 9, 2272–2280. [Google Scholar] [CrossRef] [PubMed]
- Matschke, V.; Theiss, C.; Matschke, J. Oxidative stress: The lowest common denominator of multiple diseases. Neural Regen. Res. 2019, 14, 238–241. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.L.; Norhaizan, M.E.; Liew, W.P.; Sulaiman Rahman, H. Antioxidant and Oxidative Stress: A Mutual Interplay in Age-Related Diseases. Front. Pharmacol. 2018, 9, 1162. [Google Scholar] [CrossRef]
- Lubrano, V.; Balzan, S. Enzymatic antioxidant system in vascular inflammation and coronary artery disease. World J. Exp. Med. 2015, 5, 218–224. [Google Scholar] [CrossRef]
- Rodrigo, R.; Miranda, A.; Vergara, L. Modulation of endogenous antioxidant system by wine polyphenols in human disease. Clin. Chim. Acta 2011, 412, 410–424. [Google Scholar] [CrossRef] [PubMed]
- Maurya, A.K.; Vinayak, M. Anticarcinogenic action of quercetin by downregulation of phosphatidylinositol 3-kinase (PI3K) and protein kinase C (PKC) via induction of p53 in hepatocellular carcinoma (HepG2) cell line. Mol. Biol. Rep. 2015, 42, 1419–1429. [Google Scholar] [CrossRef] [PubMed]
- Maurya, A.K.; Vinayak, M. Modulation of PKC signaling and induction of apoptosis through suppression of reactive oxygen species and tumor necrosis factor receptor 1 (TNFR1): Key role of quercetin in cancer prevention. Tumour Biol. 2015, 36, 8913–8924. [Google Scholar] [CrossRef] [PubMed]
- Sharmila, G.; Athirai, T.; Kiruthiga, B.; Senthilkumar, K.; Elumalai, P.; Arunkumar, R.; Arunakaran, J. Chemopreventive effect of quercetin in MNU and testosterone induced prostate cancer of Sprague-Dawley rats. Nutr. Cancer 2014, 66, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Sharmila, G.; Bhat, F.A.; Arunkumar, R.; Elumalai, P.; Raja Singh, P.; Senthilkumar, K.; Arunakaran, J. Chemopreventive effect of quercetin, a natural dietary flavonoid on prostate cancer in in vivo model. Clin. Nutr. 2014, 33, 718–726. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.; Dixit, S. Quercetin attenuates the development of 7, 12-dimethyl benz (a) anthracene (DMBA) and croton oil-induced skin cancer in mice. J. Biomed. Res. 2015, 29, 139–144. [Google Scholar] [PubMed]
- Li, B.; Yang, M.; Liu, J.W.; Yin, G.T. Protective mechanism of quercetin on acute myocardial infarction in rats. Genet. Mol. Res. 2016, 15, 15017117. [Google Scholar] [CrossRef] [PubMed]
- Jing, Z.H.; Wang, Z.R.; Li, X.J.; Li, X.T.; Cao, T.T.; Bi, Y.; Zhou, J.C.; Chen, X.; Yu, D.Q.; Zhu, L.; et al. Protective Effect of Quercetin on Posttraumatic Cardiac Injury. Sci. Rep. 2016, 6, 30812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mehta, V.; Parashar, A.; Udayabanu, M. Quercetin prevents chronic unpredictable stress induced behavioral dysfunction in mice by alleviating hippocampal oxidative and inflammatory stress. Physiol. Behav. 2017, 171, 69–78. [Google Scholar] [CrossRef]
- Holzmann, I.; da Silva, L.M.; Correa da Silva, J.A.; Steimbach, V.M.; de Souza, M.M. Antidepressant-like effect of quercetin in bulbectomized mice and involvement of the antioxidant defenses, and the glutamatergic and oxidonitrergic pathways. Pharmacol. Biochem. Behav. 2015, 136, 55–63. [Google Scholar] [CrossRef]
- Sherif, I.O. Uroprotective mechanism of quercetin against cyclophosphamide-induced urotoxicity: Effect on oxidative stress and inflammatory markers. J. Cell. Biochem. 2018, 119, 7441–7448. [Google Scholar] [CrossRef] [PubMed]
- Maksymchuk, O.; Shysh, A.; Rosohatska, I.; Chashchyn, M. Quercetin prevents type 1 diabetic liver damage through inhibition of CYP2E1. Pharmacol. Rep. 2017, 69, 1386–1392. [Google Scholar] [CrossRef] [PubMed]
- Adedara, I.A.; Subair, T.I.; Ego, V.C.; Oyediran, O.; Farombi, E.O. Chemoprotective role of quercetin in manganese-induced toxicity along the brain-pituitary-testicular axis in rats. Chem. Biol. Interact. 2017, 263, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Taslidere, E.; Dogan, Z.; Elbe, H.; Vardi, N.; Cetin, A.; Turkoz, Y. Quercetin protection against ciprofloxacin induced liver damage in rats. Biotech. Histochem. 2016, 91, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Miao, M.; Zhang, Y.; Liu, R.; Li, X.; Cui, Y.; Qu, L. Quercetin ameliorates liver injury induced with Tripterygium glycosides by reducing oxidative stress and inflammation. Can. J. Physiol. Pharmacol. 2015, 93, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Unsal, C.; Kanter, M.; Aktas, C.; Erboga, M. Role of quercetin in cadmium-induced oxidative stress, neuronal damage, and apoptosis in rats. Toxicol. Ind. Health 2015, 31, 1106–1115. [Google Scholar] [CrossRef] [PubMed]
- Olayinka, E.T.; Ore, A.; Adeyemo, O.A.; Ola, O.S.; Olotu, O.O.; Echebiri, R.C. Quercetin, a Flavonoid Antioxidant, Ameliorated Procarbazine-Induced Oxidative Damage to Murine Tissues. Antioxidants 2015, 4, 304–321. [Google Scholar] [CrossRef] [Green Version]
- Jahan, S.; Iftikhar, N.; Ullah, H.; Rukh, G.; Hussain, I. Alleviative effect of quercetin on rat testis against arsenic: A histological and biochemical study. Syst. Biol. Reprod. Med. 2015, 61, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.S.; Zhong, T.; Wu, H. Quercetin protects against lipopolysaccharide-induced acute lung injury in rats through suppression of inflammation and oxidative stress. Arch. Med. Sci. 2015, 11, 427–432. [Google Scholar] [CrossRef] [Green Version]
- Almaghrabi, O.A. Molecular and biochemical investigations on the effect of quercetin on oxidative stress induced by cisplatin in rat kidney. Saudi J. Biol. Sci. 2015, 22, 227–231. [Google Scholar] [CrossRef]
- Yazici, S.; Ozcan, C.U.; Hismiogullari, A.A.; Sunay, F.B.; Ozcan, T.; Berksoy, E.A.; Aksoz, E. Protective Effects of Quercetin on Necrotizing Enterocolitis in a Neonatal Rat Model. Am. J. Perinatol. 2018, 35, 434–440. [Google Scholar]
- Yao, Z.; Gu, Y.; Zhang, Q.; Liu, L.; Meng, G.; Wu, H.; Xia, Y.; Bao, X.; Shi, H.; Sun, S.; et al. Estimated daily quercetin intake and association with the prevalence of type 2 diabetes mellitus in Chinese adults. Eur. J. Nutr. 2018. [Google Scholar] [CrossRef] [PubMed]
- Veith, C.; Drent, M.; Bast, A.; van Schooten, F.J.; Boots, A.W. The disturbed redox-balance in pulmonary fibrosis is modulated by the plant flavonoid quercetin. Toxicol. Appl. Pharmacol. 2017, 336, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Farrag, Y.; Ide, W.; Montero, B.; Rico, M.; Rodriguez-Llamazares, S.; Barral, L.; Bouza, R. Preparation of starch nanoparticles loaded with quercetin using nanoprecipitation technique. Int. J. Biol. Macromol. 2018, 114, 426–433. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.S.; Wang, X.H.; Cai, S.F.; Liu, X.L.; Zhang, Y.F.; Yang, L.; Wang, C.; Yang, R. Quercetin nanoparticles with enhanced bioavailability as multifunctional agents toward amyloid induced neurotoxicity. J. Mater. Chem. B 2018, 6, 1387–1393. [Google Scholar] [CrossRef]
- Aghapour, F.; Moghadamnia, A.A.; Nicolini, A.; Kani, S.N.M.; Barari, L.; Morakabati, P.; Rezazadeh, L.; Kazemi, S. Quercetin conjugated with silica nanoparticles inhibits tumor growth in MCF-7 breast cancer cell lines. Biochem. Biophys. Res. Commun. 2018, 500, 860–865. [Google Scholar] [CrossRef] [PubMed]
- Vijayakumar, A.; Baskaran, R.; Jang, Y.S.; Oh, S.H.; Yoo, B.K. Quercetin-Loaded Solid Lipid Nanoparticle Dispersion with Improved Physicochemical Properties and Cellular Uptake. AAPS PharmSciTech 2017, 18, 875–883. [Google Scholar] [CrossRef]
- Patra, A.; Satpathy, S.; Shenoy, A.K.; Bush, J.A.; Kazi, M.; Hussain, M.D. Formulation and evaluation of mixed polymeric micelles of quercetin for treatment of breast, ovarian, and multidrug resistant cancers. Int. J. Nanomed. 2018, 13, 2869–2881. [Google Scholar] [CrossRef]
- Ahmad, N.; Ahmad, R.; Naqvi, A.A.; Alam, M.A.; Ashafaq, M.; Abdur Rub, R.; Ahmad, F.J. Intranasal delivery of quercetin-loaded mucoadhesive nanoemulsion for treatment of cerebral ischaemia. Artif. Cells Nanomed. Biotechnol. 2018, 46, 717–729. [Google Scholar] [CrossRef]
- Ramadon, D.; Anwar, E.; Harahap, Y. In vitro Penetration and Bioavailability of Novel Transdermal Quercetin-loaded Ethosomal Gel. Indian J. Pharm. Sci. 2017, 79, 948–956. [Google Scholar] [CrossRef]
- Khor, C.M.; Ng, W.K.; Chan, K.P.; Dong, Y. Preparation and characterization of quercetin/dietary fiber nanoformulations. Carbohydr. Polym. 2017, 161, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Wang, Q.; Li, T.; Xia, N.; Xia, Q. Nanostructured lipid carrier (NLC) as a strategy for encapsulation of quercetin and linseed oil: Preparation and in vitro characterization studies. J. Food Eng. 2017, 215, 1–12. [Google Scholar] [CrossRef]


| Inductive Factors | Damage Name | Protection Mechanisms | Result |
|---|---|---|---|
| LPS/d-GalN | Acute liver injury | Inhibits the activation of NF-κB and MAPK signaling pathways and inhibits the expression of apoptosis-related proteins induced by LPS/d-GalN | Decreases the production of LPS/d-GalN induced by oxidation markers [33] |
| Toosendanin | Liver toxicity | Induces Nrf2/GCL/GSH antioxidant signal transduction pathways | Increases Nrf2-mediated GCLC/GCLM expression, thereby increasing GSH content in cells [34] |
| Alcohol | Liver damage | Regulates phosphoinositide 3-kinase/Akt/NF-κB and STAT3 pathways | Enhances the body’s antioxidant, anti-inflammatory, and anti-apoptotic effects [35] |
| A variety of liver toxins | Liver toxicity | Induces p62 expression and inhibits the binding of Keap1 and Nrf2 | Increases the transcription expression of Nrf2-targeted antioxidant genes [36] |
| Doxorubicin | Heart toxicity | Upregulates Bmi-1 expression to reduce oxidative stress | Reduces DNA damage at ROS levels and maintains cardiomyocyte viability [37] |
| CCl4 | Liver damage | Improves antioxidant activity and regulates TLR2/TLR4 and MAPK/NF-κB signaling pathways | Inhibits ROS production in the liver and attenuates CCl4-induced oxidative damage [38] |
| Lead | Liver damage | Reduces oxidative stress in liver, inhibits JNK phosphorylation, and increases PI3K and Akt levels | Effectively inhibits lead-induced endoplasmic reticulum stress [39] |
| Cadmium | Cerebral cholinergic dysfunction | Reduces the production of ROS and protects the integrity of the line by regulating the protein involved in apoptosis and MAPK signal conduction | Regulates molecular targets involved in the signal conduction of brain cholinergic energy and reduces the neurotoxicity of cadmium [40] |
| Malignant cell transformation | Protects BEAS-2B cells from Cr (VI) induction by targeting miR-21-PDCD4 signaling | Reduces ROS production induced by Cr (VI) exposure in BEAS-2B cells [41] | |
| d-lactose | Cognitive impairment and neuron degeneration or loss | Improves the Nrf2-ARE signaling pathway | Decreases free radicals, increases antioxidant enzyme activity, improves overall antioxidant capacity, and slows down aging by improving Nrf2 [42] |
| Receptor activator for NF-κB ligand | Osteoblast differentiation | Regulates the transcription activities of NF-κB and AP-1 | Inhibits the NF-κB and AP-1 mechanism activation [43] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, D.; Hu, M.-J.; Wang, Y.-Q.; Cui, Y.-L. Antioxidant Activities of Quercetin and Its Complexes for Medicinal Application. Molecules 2019, 24, 1123. https://doi.org/10.3390/molecules24061123
Xu D, Hu M-J, Wang Y-Q, Cui Y-L. Antioxidant Activities of Quercetin and Its Complexes for Medicinal Application. Molecules. 2019; 24(6):1123. https://doi.org/10.3390/molecules24061123
Chicago/Turabian StyleXu, Dong, Meng-Jiao Hu, Yan-Qiu Wang, and Yuan-Lu Cui. 2019. "Antioxidant Activities of Quercetin and Its Complexes for Medicinal Application" Molecules 24, no. 6: 1123. https://doi.org/10.3390/molecules24061123