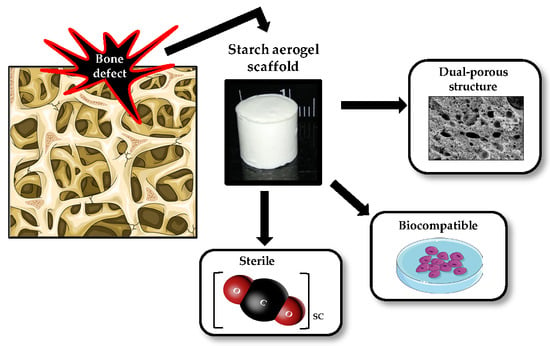
Sterile and Dual-Porous Aerogels Scaffolds Obtained through a Multistep Supercritical CO2-Based Approach
Abstract
:1. Introduction
2. Results and Discussion
2.1. Starch-Based Aerogels Preparation
2.2. Morphological and Physicochemical Characterization of the Starch Aerogels
2.3. Supercritical CO2 Sterilization Treatment Efficacy and Influence on Aerogel Properties
2.4. Cell Viability Assay
3. Materials and Methods
3.1. Materials
3.2. Starch Aerogels Processing
3.3. Supercritical CO2 Sterilization Treatment
3.4. Physicochemical, Structural and Mechanical Characterization
3.5. Microbiological Assessment
3.6. Cell Viability Assay
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- García-González, C.A.; Concheiro, A.; Alvarez-Lorenzo, C. Processing of Materials for Regenerative Medicine Using Supercritical Fluid Technology. Bioconjug. Chem. 2015, 26, 1159–1171. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, F.J. Biomaterials & scaffolds for tissue engineering. Mater. Today 2011, 14, 88–95. [Google Scholar]
- Ma, P.X. Scaffolds for tissue fabrication. Mater. Today 2004, 7, 30–40. [Google Scholar] [CrossRef]
- Martins, M.; Barros, A.A.; Quraishi, S.; Gurikov, P.; Raman, S.P.; Smirnova, I.; Duarte, A.R.C.; Reis, R.L. Preparation of macroporous alginate-based aerogels for biomedical applications. J. Supercrit. Fluids 2015, 106, 152–159. [Google Scholar] [CrossRef] [Green Version]
- García-González, C.A.; Alnaief, M.; Smirnova, I. Polysaccharide-based aerogels—Promising biodegradable carriers for drug delivery systems. Carbohydr. Polym. 2011, 86, 1425–1438. [Google Scholar] [CrossRef]
- Maleki, H.; Durães, L.; García-González, C.A.; del Gaudio, P.; Portugal, A.; Mahmoudi, M. Synthesis and biomedical applications of aerogels: Possibilities and challenges. Adv. Colloid Interface Sci. 2016, 236, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Kistler, S.S. Coherent Expanded Aerogels and Jellies. Nature 1931, 127, 741. [Google Scholar] [CrossRef]
- Pierre, A.C.; Pajonk, G.M. Chemistry of Aerogels and Their Applications. Chem. Rev. 2002, 102, 4243–4266. [Google Scholar] [CrossRef] [PubMed]
- Nardecchia, S.; Carriazo, D.; Ferrer, M.L.; Gutiérrez, M.C.; del Monte, F. Three dimensional macroporous architectures and aerogels built of carbon nanotubes and/or graphene: Synthesis and applications. Chem. Soc. Rev. 2013, 42, 794–830. [Google Scholar] [CrossRef] [PubMed]
- Hrubesh, L.W. Aerogel applications. J. Non-Cryst. Solids 1998, 225, 335–342. [Google Scholar] [CrossRef]
- Guo, X.; Shan, J.; Lai, Z.; Lei, W.; Ding, R.; Zhang, Y.; Yang, H. Facile Synthesis of Flexible Methylsilsesquioxane Aerogels with Surface Modifications for Sound- Absorbance, Fast Dye Adsorption and Oil/Water Separation. Molecules 2018, 23, 945. [Google Scholar] [CrossRef] [PubMed]
- Druel, L.; Bardl, R.; Vorwerg, W.; Budtova, T. Starch Aerogels: A Member of the Family of Thermal Superinsulating Materials. Biomacromolecules 2017, 18, 4232–4239. [Google Scholar] [CrossRef] [PubMed]
- García-González, C.A.; López-Iglesias, C.; Concheiro, A.; Alvarez-Lorenzo, C. Chapter 16. Biomedical Applications of Polysaccharide and Protein Based Aerogels. In Green Chemistry Series; Thomas, S., Pothan, L.A., Mavelil-Sam, R., Eds.; Royal Society of Chemistry: Cambridge, UK, 2018; pp. 295–323. ISBN 978-1-78262-765-4. [Google Scholar]
- Silva, S.S.; Duarte, A.R.C.; Carvalho, A.P.; Mano, J.F.; Reis, R.L. Green processing of porous chitin structures for biomedical applications combining ionic liquids and supercritical fluid technology. Acta Biomater. 2011, 7, 1166–1172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goimil, L.; Braga, M.E.M.; Dias, A.M.A.; Gómez-Amoza, J.L.; Concheiro, A.; Alvarez-Lorenzo, C.; de Sousa, H.C.; García-González, C.A. Supercritical processing of starch aerogels and aerogel-loaded poly(ε-caprolactone) scaffolds for sustained release of ketoprofen for bone regeneration. J. CO2 Util. 2017, 18, 237–249. [Google Scholar] [CrossRef]
- Ganesan, K.; Dennstedt, A.; Barowski, A.; Ratke, L. Design of aerogels, cryogels and xerogels of cellulose with hierarchical porous structures. Mater. Des. 2016, 92, 345–355. [Google Scholar] [CrossRef]
- García-González, C.A.; Uy, J.J.; Alnaief, M.; Smirnova, I. Preparation of tailor-made starch-based aerogel microspheres by the emulsion-gelation method. Carbohydr. Polym. 2012, 88, 1378–1386. [Google Scholar] [CrossRef]
- Silva, G.A.; Coutinho, O.P.; Ducheyne, P.; Shapiro, I.M.; Reis, R.L. The effect of starch and starch-bioactive glass composite microparticles on the adhesion and expression of the osteoblastic phenotype of a bone cell line. Biomaterials 2007, 28, 326–334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, A.; Zhou, B.; Zhang, Z.; Shen, J. A Special Material or a New State of Matter: A Review and Reconsideration of the Aerogel. Materials 2013, 6, 941–968. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hutmacher, D.W. Scaffolds in tissue engineering bone and cartilage. Biomaterials 2000, 21, 2529–2543. [Google Scholar] [CrossRef]
- Zhang, R.; Ma, P.X. Synthetic nano-fibrillar extracellular matrices with predesigned macroporous architectures. J. Biomed. Mater. Res. 2000, 52, 430–438. [Google Scholar] [CrossRef]
- Reverchon, E.; Cardea, S.; Rapuano, C. A new supercritical fluid-based process to produce scaffolds for tissue replacement. J. Supercrit. Fluids 2008, 45, 365–373. [Google Scholar] [CrossRef]
- Baldino, L.; Naddeo, F.; Cardea, S.; Naddeo, A.; Reverchon, E. FEM modeling of the reinforcement mechanism of Hydroxyapatite in PLLA scaffolds produced by supercritical drying, for Tissue Engineering applications. J. Mech. Behav. Biomed. Mater. 2015, 51, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Gao, C.; Gong, Y.; Shen, J. Paraffin spheres as porogen to fabricate poly(L-lactic acid) scaffolds with improved cytocompatibility for cartilage tissue engineering. J. Biomed. Mater. Res. 2003, 67B, 610–617. [Google Scholar] [CrossRef] [PubMed]
- Shukla, R.; Cheryan, M. Zein: The industrial protein from corn. Ind. Crops Prod. 2001, 13, 171–192. [Google Scholar] [CrossRef]
- Dong, J.; Sun, Q.; Wang, J.-Y. Basic study of corn protein, zein, as a biomaterial in tissue engineering, surface morphology and biocompatibility. Biomaterials 2004, 25, 4691–4697. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Gong, S.; Lin, Z.; Fu, J.; Xue, S.; Huang, J.; Wang, J. In vivo biocompatibility and mechanical properties of porous zein scaffolds. Biomaterials 2007, 28, 3952–3964. [Google Scholar] [CrossRef] [PubMed]
- Tu, J.; Wang, H.; Li, H.; Dai, K.; Wang, J.; Zhang, X. The in vivo bone formation by mesenchymal stem cells in zein scaffolds. Biomaterials 2009, 30, 4369–4376. [Google Scholar] [CrossRef] [PubMed]
- Sandhu, K.; Singh, N. Some properties of corn starches II: Physicochemical, gelatinization, retrogradation, pasting and gel textural properties. Food Chem. 2007, 101, 1499–1507. [Google Scholar] [CrossRef]
- Dai, Z.; Ronholm, J.; Tian, Y.; Sethi, B.; Cao, X. Sterilization techniques for biodegradable scaffolds in tissue engineering applications. J. Tissue Eng. 2016, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Davis, T.A.; Matthews, M.A.; Drews, M.J.; LaBerge, M.; An, Y.H. Sterilization using high-pressure carbon dioxide. J. Supercrit. Fluids 2006, 38, 354–372. [Google Scholar] [CrossRef]
- Karajanagi, S.S.; Yoganathan, R.; Mammucari, R.; Park, H.; Cox, J.; Zeitels, S.M.; Langer, R.; Foster, N.R. Application of a dense gas technique for sterilizing soft biomaterials. Biotechnol. Bioeng. 2011, 108, 1716–1725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García-González, C.A.; Smirnova, I. Use of supercritical fluid technology for the production of tailor-made aerogel particles for delivery systems. J. Supercrit. Fluids 2013, 79, 152–158. [Google Scholar] [CrossRef]
- Lawton, J.W. Isolation of Zein Using 100% Ethanol. Cereal Chem. J. 2006, 83, 565–568. [Google Scholar] [CrossRef]
- Buchtová, N.; Budtova, T. Cellulose aero-, cryo- and xerogels: Towards understanding of morphology control. Cellulose 2016, 23, 2585–2595. [Google Scholar] [CrossRef]
- Zamora-Sequeira, R.; Ardao, I.; Starbird, R.; García-González, C.A. Conductive nanostructured materials based on poly-(3,4-ethylenedioxythiophene) (PEDOT) and starch/κ-carrageenan for biomedical applications. Carbohydr. Polym. 2018, 189, 304–312. [Google Scholar] [CrossRef] [PubMed]
- Athanasiou, K.A.; Zhu, C.-F.; Lanctot, D.R.; Agrawal, C.M.; Wang, X. Fundamentals of Biomechanics in Tissue Engineering of Bone. Tissue Eng. 2000, 6, 361–381. [Google Scholar] [CrossRef] [PubMed]
- Kenar, J.A.; Eller, F.J.; Felker, F.C.; Jackson, M.A.; Fanta, G.F. Starch aerogel beads obtained from inclusion complexes prepared from high amylose starch and sodium palmitate. Green Chem 2014, 16, 1921–1930. [Google Scholar] [CrossRef]
- Zhang, J.; Wen, C.; Zhang, H.; Zandile, M.; Luo, X.; Duan, Y.; Ma, H. Structure of the zein protein as treated with subcritical water. Int. J. Food Prop. 2018, 21, 128–138. [Google Scholar] [CrossRef] [Green Version]
- Cabra, V.; Arreguin, R.; Vazquez-Duhalt, R.; Farres, A. Effect of temperature and pH on the secondary structure and processes of oligomerization of 19 kDa alpha-zein. Biochim. Biophys. Acta BBA Proteins Proteomics 2006, 1764, 1110–1118. [Google Scholar] [CrossRef] [PubMed]
- Pascoli, M.; de Lima, R.; Fraceto, L.F. Zein Nanoparticles and Strategies to Improve Colloidal Stability: A Mini-Review. Front. Chem. 2018, 6, 6. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Dai, L.; Liu, F.; Gao, Y. Simultaneous treatment of heat and high pressure homogenization of zein in ethanol–water solution: Physical, structural, thermal and morphological characteristics. Innov. Food Sci. Emerg. Technol. 2016, 34, 161–170. [Google Scholar] [CrossRef]
- Vagaská, B.; Bacáková, L.; Filová, E.; Balík, K. Osteogenic cells on bio-inspired materials for bone tissue engineering. Physiol. Res. 2010, 59, 309–322. [Google Scholar] [PubMed]
- Gentile, F.; La Rocca, R.; Marinaro, G.; Nicastri, A.; Toma, A.; Paonessa, F.; Cojoc, G.; Liberale, C.; Benfenati, F.; di Fabrizio, E.; et al. Differential Cell Adhesion on Mesoporous Silicon Substrates. ACS Appl. Mater. Interfaces 2012, 4, 2903–2911. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Elkhooly, T.A.; Huang, Q.; Liu, X.; Yang, X.; Yan, H.; Xiong, Z.; Ma, J.; Feng, Q.; Shen, Z. Effects of the hierarchical macro/mesoporous structure on the osteoblast-like cell response. J. Biomed. Mater. Res. A 2018, 106, 1896–1902. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, X.; Jiang, X.; Yamaguchi, M.; Ito, A.; Bando, Y.; Golberg, D. Boron nitride nanotube-enhanced osteogenic differentiation of mesenchymal stem cells. J. Biomed. Mater. Res. B Appl. Biomater. 2016, 104, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, N.; Kanazawa, M.; Tsuru, K.; Tsuchiya, A.; Sunarso; Toita, R.; Mori, Y.; Nakashima, Y.; Ishikawa, K. Synergistic effect of surface phosphorylation and micro-roughness on enhanced osseointegration ability of poly(ether ether ketone) in the rabbit tibia. Sci. Rep. 2018, 8, 16887. [Google Scholar] [CrossRef] [PubMed]
- Mendonça, G.; Mendonça, D.B.S.; Aragão, F.J.L.; Cooper, L.F. Advancing dental implant surface technology—From micron- to nanotopography. Biomaterials 2008, 29, 3822–3835. [Google Scholar] [CrossRef] [PubMed]
- Rønold, H.J.; Ellingsen, J.E. Effect of micro-roughness produced by TiO2 blasting--tensile testing of bone attachment by using coin-shaped implants. Biomaterials 2002, 23, 4211–4219. [Google Scholar] [CrossRef]
- Pircher, N.; Fischhuber, D.; Carbajal, L.; Strauß, C.; Nedelec, J.-M.; Kasper, C.; Rosenau, T.; Liebner, F. Preparation and Reinforcement of Dual-Porous Biocompatible Cellulose Scaffolds for Tissue Engineering. Macromol. Mater. Eng. 2015, 300, 911–924. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García-González, C.A.; Fraile, J.; López-Periago, A.; Domingo, C. Preparation of silane-coated TiO2 nanoparticles in supercritical CO2. J. Colloid Interface Sci. 2009, 338, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Tkalec, G.; Pantić, M.; Novak, Z.; Knez, Ž. Supercritical impregnation of drugs and supercritical fluid deposition of metals into aerogels. J. Mater. Sci. 2015, 50, 1–12. [Google Scholar] [CrossRef]
- Li, Y.; He, Z.-D.; Zheng, Q.-E.; Hu, C.; Lai, W.-F. Hydroxypropyl-β-cyclodextrin for Delivery of Baicalin via Inclusion Complexation by Supercritical Fluid Encapsulation. Molecules 2018, 23, 1169. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Burrows, S.; Gleason, C.; Matthews, M.A.; Drews, M.J.; LaBerge, M.; An, Y.H. Sterilizing Bacillus pumilus spores using supercritical carbon dioxide. J. Microbiol. Methods 2006, 66, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Özbakır, Y.; Erkey, C. Experimental and theoretical investigation of supercritical drying of silica alcogels. J. Supercrit. Fluids 2015, 98, 153–166. [Google Scholar] [CrossRef]
- García-González, C.A.; Camino-Rey, M.C.; Alnaief, M.; Zetzl, C.; Smirnova, I. Supercritical drying of aerogels using CO2: Effect of extraction time on the end material textural properties. J. Supercrit. Fluids 2012, 66, 297–306. [Google Scholar] [CrossRef]
- King, M.B.; Mubarak, A.; Kim, J.D.; Bott, T.R. The mutual solubilities of water with supercritical and liquid carbon dioxides. J. Supercrit. Fluids 1992, 5, 296–302. [Google Scholar] [CrossRef]
- García-González, C.A.; Barros, J.; Rey-Rico, A.; Redondo, P.; Gómez-Amoza, J.L.; Concheiro, A.; Alvarez-Lorenzo, C.; Monteiro, F.J. Antimicrobial Properties and Osteogenicity of Vancomycin-Loaded Synthetic Scaffolds Obtained by Supercritical Foaming. ACS Appl. Mater. Interfaces 2018, 10, 3349–3360. [Google Scholar] [CrossRef] [PubMed]
- Marques, A.P.; Reis, R.L.; Hunt, J.A. The biocompatibility of novel starch-based polymers and composites: In vitro studies. Biomaterials 2002, 23, 1471–1478. [Google Scholar] [CrossRef]
- Flores-Arriaga, J.C.; de Jesús Pozos-Guillén, A.; Escobar-García, D.M.; Grandfils, C.; Cerda-Cristerna, B.I. Cell viability and hemocompatibility evaluation of a starch-based hydrogel loaded with hydroxyapatite or calcium carbonate for maxillofacial bone regeneration. Odontology 2017, 105, 398–407. [Google Scholar] [CrossRef] [PubMed]
- Şahin, İ.; Özbakır, Y.; İnönü, Z.; Ulker, Z.; Erkey, C. Kinetics of Supercritical Drying of Gels. Gels 2017, 4, 3. [Google Scholar] [CrossRef] [PubMed]
- Hemmer, J.D.; Drews, M.J.; LaBerge, M.; Matthews, M.A. Sterilization of bacterial spores by using supercritical carbon dioxide and hydrogen peroxide. J. Biomed. Mater. Res. B Appl. Biomater. 2007, 80B, 511–518. [Google Scholar] [CrossRef] [PubMed]
- Bernhardt, A.; Wehrl, M.; Paul, B.; Hochmuth, T.; Schumacher, M.; Schütz, K.; Gelinsky, M. Improved Sterilization of Sensitive Biomaterials with Supercritical Carbon Dioxide at Low Temperature. PLoS ONE 2015, 10, e0129205. [Google Scholar] [CrossRef] [PubMed]
- ISO 17665-1:2006. Sterilization of Health CARE products—Moist Heat—Part 1: Requirements for the Development, Validation and Routine Control of a Sterilization Process for Medical Devices; ISO: Geneva, Switzerland, 2006. [Google Scholar]
- ISO 11135:2014. Sterilization of Health-Care Products—Ethylene Oxide—Requirements for the Development, Validation and Routine Control of a Sterilization Process for Medical Devices; ISO: Geneva, Switzerland, 2014. [Google Scholar]
- ISO 11137-1:2006/Amd.1:2013. Sterilization of Health Care Products—Radiation—Part 1: Requirements for Development, Validation and Routine Control of a Sterilization Process for MEDICAL devices; ISO: Geneva, Switzerland, 2006. [Google Scholar]
Sample Availability: Samples of the starch aerogels are available from the authors. |








| Aerogel | ρBulk (g/cm3) | ρSkel (g/cm3) | ε (%) | ABET (m2/g) | Vp,BJH (cm3/g) | dp,BJH (nm) | Vp (cm3/g) | Vp,meso (%) | Vp,lmac (%) | Vp,macro (%) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| [Z0] | 0.166 ± 0.003 | 1.490 ± 0.059 | 88.9 ± 0.2 | 39.1 ± 2.7 | 183 ± 9 | 1.01 ± 0.05 | 19.1 ± 1.0 | 5.35 | 14.2 | 4.7 | 85.8 |
| [Z0]S | 0.304 ± 0.009 | 1.541 ± 0.045 | 80.3 ± 0.6 | 65.8 ± 2.2 | 130 ± 6 | 0.84 ± 0.04 | 22.9 ± 1.2 | 2.64 | 20.8 | 10.9 | 79.2 |
| [Z1] | 0.135 ± 0.005 | 1.498 ± 0.059 | 91.0 ± 0.3 | 25.2 ± 5.0 | 208 ± 10 | 0.98 ± 0.05 | 16.5 ± 0.8 | 6.74 | 10.9 | 3.5 | 89.1 |
| [Z1]S | 0.203 ± 0.016 | 1.512 ± 0.038 | 86.6 ± 1.1 | 46.6 ± 7.9 | 264 ± 13 | 1.25 ± 0.06 | 16.1 ± 0.8 | 4.26 | 23.6 | 4.9 | 76.4 |
| [Z2] | 0.189 ± 0.007 | 1.423 ± 0.055 | 86.7 ± 0.5 | 21.7 ± 4.3 | 143 ± 7 | 0.74 ± 0.04 | 16.9 ± 0.8 | 4.59 | 12.3 | 3.6 | 87.7 |
| [Z2]S | 0.214 ± 0.016 | 1.431 ± 0.065 | 85.1 ± 1.0 | 25.2 ± 3.5 | 146 ± 7 | 1.00 ± 0.05 | 21.7 ± 1.1 | 3.97 | 16.7 | 8.2 | 83.3 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos-Rosales, V.; Ardao, I.; Alvarez-Lorenzo, C.; Ribeiro, N.; Oliveira, A.L.; García-González, C.A. Sterile and Dual-Porous Aerogels Scaffolds Obtained through a Multistep Supercritical CO2-Based Approach. Molecules 2019, 24, 871. https://doi.org/10.3390/molecules24050871
Santos-Rosales V, Ardao I, Alvarez-Lorenzo C, Ribeiro N, Oliveira AL, García-González CA. Sterile and Dual-Porous Aerogels Scaffolds Obtained through a Multistep Supercritical CO2-Based Approach. Molecules. 2019; 24(5):871. https://doi.org/10.3390/molecules24050871
Chicago/Turabian StyleSantos-Rosales, Víctor, Inés Ardao, Carmen Alvarez-Lorenzo, Nilza Ribeiro, Ana L. Oliveira, and Carlos A. García-González. 2019. "Sterile and Dual-Porous Aerogels Scaffolds Obtained through a Multistep Supercritical CO2-Based Approach" Molecules 24, no. 5: 871. https://doi.org/10.3390/molecules24050871