HMPA-Catalyzed Transfer Hydrogenation of 3-Carbonyl Pyridines and Other N-Heteroarenes with Trichlorosilane
Abstract
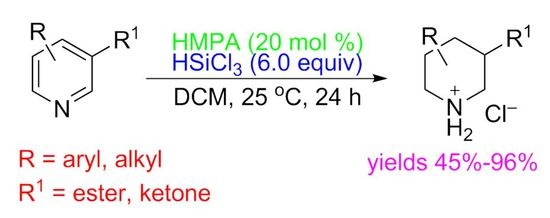
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Reduction of Pyridines and N-Heteroaromatics
3.2. The Stereochemical Assignment of Disubstituted Piperidines
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Källström, S.; Leino, R. Synthesis of pharmaceutically active compounds containing a disubstituted piperidine framework. Bioorg. Med. Chem. 2008, 16, 601–635. [Google Scholar] [CrossRef]
- Cadu, A.; Upadhyay, P.K.; Andersson, P.G. Iridium-Catalyzed Asymmetric Hydrogenation of Substituted Pyridines. Asian J. Org. Chem. 2013, 2, 1061–1065. [Google Scholar] [CrossRef] [Green Version]
- Chang, M.; Huang, Y.; Liu, S.; Chen, Y.; Krska, S.W.; Davies, I.W.; Zhang, X. Asymmetric Hydrogenation of Pyridinium Salts with an Iridium Phosphole Catalyst. Angew. Chem. Int. Ed. 2014, 53, 12761–12764. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.-W.; Ye, Z.-S.; Chen, Z.-P.; Wu, B.; Zhou, Y.-G. Enantioselective synthesis of trifluoromethyl substituted piperidines with multiple stereogenic centers via hydrogenation of pyridinium hydrochlorides. Org. Chem. Front. 2015, 2, 586–589. [Google Scholar] [CrossRef]
- Glorius, F. Asymmetric hydrogenation of aromatic compounds. Org. Biomol. Chem. 2005, 3, 4171–4175. [Google Scholar] [CrossRef] [PubMed]
- Glorius, F.; Spielkamp, N.; Holle, S.; Goddard, R.; Lehmann, C.W. Efficient Asymmetric Hydrogenation of Pyridines. Angew. Chem. Int. Ed. 2004, 43, 2850–2852. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.-X.; Wu, B.; Gao, X.; Chen, M.-W.; Wang, B.; Zhou, Y.-G. Iridium-Catalyzed Selective Hydrogenation of 3-Hydroxypyridinium Salts: A Facile Synthesis of Piperidin-3-ones. Org. Lett. 2015, 17, 1640–1643. [Google Scholar] [CrossRef]
- Huang, W.-X.; Yu, C.-B.; Ji, Y.; Liu, L.-J.; Zhou, Y.-G. Iridium-Catalyzed Asymmetric Hydrogenation of Heteroaromatics Bearing a Hydroxyl Group, 3-Hydroxypyridinium Salts. ACS Catal. 2016, 6, 2368–2371. [Google Scholar] [CrossRef]
- Iimuro, A.; Higashida, K.; Kita, Y.; Mashima, K. Asymmetric Hydrogenation of 3-Amido-2-arylpyridinium Salts by Triply Chloride-Bridged Dinuclear Iridium Complexes Bearing Enantiopure Diphosphine Ligands: Synthesis of Neurokinin-1 Receptor Antagonist Derivatives. Adv. Synth. Catal. 2016, 358, 1929–1933. [Google Scholar] [CrossRef]
- Karakulina, A.; Gopakumar, A.; Akçok, İ.; Roulier, B.L.; LaGrange, T.; Katsyuba, S.A.; Das, S.; Dyson, P.J. A Rhodium Nanoparticle–Lewis Acidic Ionic Liquid Catalyst for the Chemoselective Reduction of Heteroarenes. Angew. Chem. Int. Ed. 2016, 55, 292–296. [Google Scholar] [CrossRef]
- Legault, C.Y.; Charette, A.B. Catalytic Asymmetric Hydrogenation of N-Iminopyridinium Ylides: Expedient Approach to Enantioenriched Substituted Piperidine Derivatives. J. Am. Chem. Soc. 2005, 127, 8966–8967. [Google Scholar] [CrossRef] [PubMed]
- Lei, A.; Chen, M.; He, M.; Zhang, X. Asymmetric Hydrogenation of Pyridines: Enantioselective Synthesis of Nipecotic Acid Derivatives. Eur. J. Org. Chem. 2006, 2006, 4343–4347. [Google Scholar] [CrossRef]
- Oshima, K.; Ohmura, T.; Suginome, M. Regioselective Synthesis of 1,2-Dihydropyridines by Rhodium-Catalyzed Hydroboration of Pyridines. J. Am. Chem. Soc. 2012, 134, 3699–3702. [Google Scholar] [CrossRef] [PubMed]
- Renom-Carrasco, M.; Gajewski, P.; Pignataro, L.; delries, J.G.; Piarulli, U.; Gennari, C.; Lefort, L. A Mixed Ligand Approach for the Asymmetric Hydrogenation of 2-Substituted Pyridinium Salts. Adv. Synth. Catal. 2016, 358, 2589–2593. [Google Scholar] [CrossRef]
- Tang, W.; Sun, Y.; Lijin, X.; Wang, T.; Qinghua, F.; Lam, K.-H.; Chan, A.S. Highly efficient and enantioselective hydrogenation of quinolines and pyridines with Ir-Difluorphos catalyst. Org. Biomol. Chem. 2010, 8, 3464–3471. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.-J.; Tan, J.; Xu, L.-J.; Lam, K.-H.; Fan, Q.-H.; Chan ASC. Highly Enantioselective Hydrogenation of Quinoline and Pyridine Derivatives with Iridium-(P-Phos) Catalyst. Adv. Synth. Catal. 2010, 352, 1055–1062. [Google Scholar] [CrossRef]
- Wu, J.; Tang, W.; Pettman, A.; Xiao, J. Efficient and Chemoselective Reduction of Pyridines to Tetrahydropyridines and Piperidines via Rhodium-Catalyzed Transfer Hydrogenation. Adv. Synth. Catal. 2012, 355, 35–40. [Google Scholar] [CrossRef]
- Ye, Z.-S.; Chen, M.-W.; Chen, Q.-A.; Shi, L.; Duan, Y.; Zhou, Y.-G. Iridium-Catalyzed Asymmetric Hydrogenation of Pyridinium Salts. Angew. Chem. Int. Ed. 2012, 51, 10181–10184. [Google Scholar] [CrossRef]
- Zheng, C.; You, S.-L. Transfer hydrogenation with Hantzsch esters and related organic hydride donors. Chem. Soc. Rev. 2012, 41, 2498–2518. [Google Scholar] [CrossRef]
- Rossi, S.; Benaglia, M.; Massolo, E.; Raimondi, L. Organocatalytic strategies for enantioselective metal-free reductions. Catal. Sci. Technol 2014, 4, 2708–2723. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.-G. Asymmetric Hydrogenation of Heteroaromatic Compounds. Acc. Chem. Res. 2007, 40, 1357–1366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, D.-S.; Chen, Q.-A.; Lu, S.-M.; Zhou, Y.-G. Asymmetric Hydrogenation of Heteroarenes and Arenes. Chem. Rev. 2012, 112, 2557–2590. [Google Scholar] [CrossRef] [PubMed]
- Irfan, M.; Petricci, E.; Glasnov, T.N.; Taddei, M.; Kappe, C.O. Continuous Flow Hydrogenation of Functionalized Pyridines. Eur. J. Org. Chem. 2009, 2009, 1327–1334. [Google Scholar] [CrossRef]
- Miyamura, H.; Suzuki, A.; Yasukawa, T.; Kobayashi, S. Polysilane-Immobilized Rh–Pt Bimetallic Nanoparticles as Powerful Arene Hydrogenation Catalysts: Synthesis, Reactions under Batch and Flow Conditions and Reaction Mechanism. J. Am. Chem. Soc. 2018, 140, 11325–11334. [Google Scholar] [CrossRef] [PubMed]
- Mahdi, T.; del Castillo, J.N.; Stephan, D.W. Metal-Free Hydrogenation of N-Based Heterocycles. Organometallics 2013, 32, 1971–1978. [Google Scholar] [CrossRef]
- Liu, Y.; Du, H. Metal-Free Borane-Catalyzed Highly Stereoselective Hydrogenation of Pyridines. J. Am. Chem. Soc. 2013, 135, 12968–12971. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhang, L.; Meng, W.; Feng, X.; Yang, J.; Du, H. Borane-Catalyzed Transfer Hydrogenations of Pyridines with Ammonia Borane. Org. Lett. 2016, 18, 5189–5191. [Google Scholar] [CrossRef]
- Gandhamsetty, N.; Park, S.; Chang, S. Selective Silylative Reduction of Pyridines Leading to Structurally Diverse Azacyclic Compounds with the Formation of sp3 C–Si Bonds. J. Am. Chem. Soc. 2015, 137, 15176–15184. [Google Scholar] [CrossRef]
- Liu, Z.-Y.; Wen, Z.-H.; Wang, X.-C. B(C6F5)3-Catalyzed Cascade Reduction of Pyridines. Angew. Chem. Int. Ed. 2017, 56, 5817–5820. [Google Scholar] [CrossRef]
- Rueping, M.; Antonchick, A.P. Organocatalytic Enantioselective Reduction of Pyridines. Angew. Chem. Int. Ed. 2007, 46, 4562–4565. [Google Scholar] [CrossRef]
- Malkov, A.V.; Vranková, K.; Stončius, S.; Kočovský, P. Asymmetric Reduction of Imines with Trichlorosilane, Catalyzed by Sigamide, an Amino Acid-Derived Formamide: Scope and Limitations. J. Org. Chem. 2009, 74, 5839–5849. [Google Scholar] [CrossRef] [PubMed]
- Guizzetti, S.; Benaglia, M. Trichlorosilane-Mediated Stereoselective Reduction of C=N Bonds. Eur. J. Org. Chem. 2010, 2010, 5529–5541. [Google Scholar] [CrossRef]
- Jones, S.; Zhao, P. Evaluating dynamic kinetic resolution strategies in the asymmetric hydrosilylation of cyclic ketimines. Tetrahedron Asymmetry 2014, 25, 238–244. [Google Scholar] [CrossRef]
- Orlandi, M.; Tosi, F.; Bonsignore, M.; Benaglia, M. Metal-Free Reduction of Aromatic and Aliphatic Nitro Compounds to Amines: A HSiCl3-Mediated Reaction of Wide General Applicability. Org. Lett. 2015, 17, 3941–3943. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.-Y.; Zhang, M.-M.; Shu, C.; Zhang, Y.-H.; Liao, L.-H.; Yuan, W.-C.; Zhang, X.-M. Enantioselective Lewis-Base-Catalyzed Asymmetric Hydrosilylation of Substituted Benzophenone N-Aryl Imines: Efficient Synthesis of Chiral (Diarylmethyl)amines. Adv. Synth. Catal. 2014, 356, 3539–3544. [Google Scholar] [CrossRef]
- Barrulas, P.C.; Genoni, A.; Benaglia, M.; Burke, A.J. Cinchona-Derived Picolinamides: Effective Organocatalysts for Stereoselective Imine Hydrosilylation. Eur. J. Org. Chem. 2014, 2014, 7339–7342. [Google Scholar] [CrossRef] [Green Version]
- Warner, C.J.A.; Reeder, A.T.; Jones, S. P-Chiral phosphine oxide catalysed reduction of prochiral ketimines using trichlorosilane. Tetrahedron Asymmetry 2016, 27, 136–141. [Google Scholar] [CrossRef] [Green Version]
- Ye, J.; Wang, C.; Chen, L.; Wu, X.; Zhou, L.; Sun, J. Chiral Lewis Base-Catalyzed, Enantioselective Reduction of Unprotected β-Enamino Esters with Trichlorosilane. Adv. Synth. Catal. 2016, 358, 1042–1047. [Google Scholar] [CrossRef]
- Kippo, T.; Hamaoka, K.; Ueda, M.; Fukuyama, T.; Ryu, I. Bromoallylation of Alkenes Leading to 4-Alkenyl Bromides Based on Trapping of β-Bromoalkyl Radicals. Org. Lett. 2017, 19, 5198–5200. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, J.; Xia, Y.-T.; Sun, X.-T.; Wu, L. Efficient Hydrogenation of Nitrogen Heterocycles Catalyzed by Carbon-Metal Covalent Bonds-Stabilized Palladium Nanoparticles: Synergistic Effects of Particle Size and Water. Adv. Synth. Catal. 2016, 358, 3039–3045. [Google Scholar] [CrossRef]
- Laval, S.; Dayoub, W.; Pehlivan, L.; Métay, E.; Favre-Reguillon, A.; Delbrayelle, D.; Mignani, G.; Lemaire, M. Straightforward access to cyclic amines by dinitriles reduction. Tetrahedron 2014, 70, 975–983. [Google Scholar] [CrossRef]
- Xuan, Q.; Song, Q. Diboron-Assisted Palladium-Catalyzed Transfer Hydrogenation of N-Heteroaromatics with Water as Hydrogen Donor and Solvent. Org. Lett. 2016, 18, 4250–4253. [Google Scholar] [CrossRef] [PubMed]
- Eisenberger, P.; Bestvater, B.P.; Keske, E.C.; Crudden, C.M. Hydrogenations at Room Temperature and Atmospheric Pressure with Mesoionic Carbene-Stabilized Borenium Catalysts. Angew. Chem. Int. Ed. 2015, 54, 1433–7851. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds 1a, 1o and 1p are available from the authors. |







| Entry | Solvent | LB (Equiv.) | Temp (°C) | Yield (%) b |
|---|---|---|---|---|
| 1 | DCM | HMPA (0.2) | 0 | 49.0 |
| 2 | DCM | DMF (0.2) | 0 | trace |
| 3 | DCM | POPh3 (0.2) | 0 | 37.0 |
| 4 | DCM | HMPA (0.2) | −10 | 38.0 |
| 5 | DCM | HMPA (0.2) | 25 | 82.0 |
| 6 | THF | HMPA (0.2) | 25 | 64.0 |
| 7 | CHCl3 | HMPA (0.2) | 25 | 76.0 |
| 8 | CCl4 | HMPA (0.2) | 25 | trace |
| 9 | DCE | HMPA (0.2) | 25 | 77.0 |
| 10 | toluene | HMPA (0.2) | 25 | trace |
| 11 | MeCN | HMPA (0.2) | 25 | trace |
| 12 c | DCM | HMPA (0.2) | 25 | 60.0 |
| 13 d | DCM | HMPA (0.2) | 25 | 81.0 |
| 14 e | DCM | HMPA (0.2) | 25 | 86.0 |
| 15 f | DCM | HMPA (0.2) | 25 | 96.0 |
| 16 f | DCM | HMPA (0.1) | 25 | 82.0 |
| 17 f | DCM | HMPA (0.05) | 25 | 54.0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, Y.; Sun, J. HMPA-Catalyzed Transfer Hydrogenation of 3-Carbonyl Pyridines and Other N-Heteroarenes with Trichlorosilane. Molecules 2019, 24, 401. https://doi.org/10.3390/molecules24030401
Fu Y, Sun J. HMPA-Catalyzed Transfer Hydrogenation of 3-Carbonyl Pyridines and Other N-Heteroarenes with Trichlorosilane. Molecules. 2019; 24(3):401. https://doi.org/10.3390/molecules24030401
Chicago/Turabian StyleFu, Yun, and Jian Sun. 2019. "HMPA-Catalyzed Transfer Hydrogenation of 3-Carbonyl Pyridines and Other N-Heteroarenes with Trichlorosilane" Molecules 24, no. 3: 401. https://doi.org/10.3390/molecules24030401