Solution Combustion Synthesis of Cr2O3 Nanoparticles and the Catalytic Performance for Dehydrofluorination of 1,1,1,3,3-Pentafluoropropane to 1,3,3,3-Tetrafluoropropene
Abstract
:1. Introduction
2. Results and Discussion
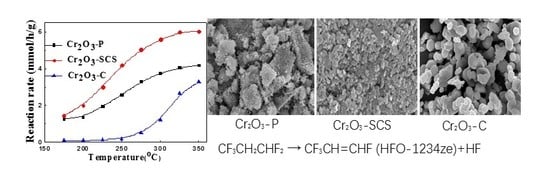
2.1. Evaluation of Catalytic Activity
2.2. Morphology and Structure of Cr2O3 Catalysts
2.3. Surface and Bulk Chemistry of Cr2O3 Prepared by Different Methods
3. Materials and Methods
3.1. Catalysts Preparation
3.1.1. Solution Combustion Synthesis Method
3.1.2. Precipitation Method
3.1.3. Commercial Cr2O3
3.2. Catalytic Activity
3.3. Catalyst Characterization
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ko, M.; Shia, R.L.; Sze, N.D.; Magid, H.; Bray, R.G. Atmospheric lifetime and global warming potential of HFC-245fa. J. Geophys. Res.-Atom. 1999, 104, 8173–8181. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.W.; Duan, Y.Y. Vapor pressures of 1,1,1,3,3-pentafluoropropane (HFC-245fa) and 1,1,1,2,3,3,3-heptafluoropropane (HFC-227ea). J. Chem. Eng. Data. 2004, 49, 1581–1585. [Google Scholar] [CrossRef]
- Quan, H.-D.; Yang, H.-E.; Tamura, M.; Sekiya, A. Preparation of 1,1,1,3,3-pentafluoropropane (HFC-245fa) by using a SbF5-attached catalyst. J. Fluorine Chem. 2007, 128, 190–195. [Google Scholar] [CrossRef]
- Vollmer, M.K.; Reimann, S.; Hill, M.; Brunner, D. First Observations of the Fourth Generation Synthetic Halocarbons HFC-1234yf, HFC-1234ze(E), and HCFC-1233zd(E) in the Atmosphere. Environ. Sci. Technol. 2015, 49, 2703–2708. [Google Scholar] [CrossRef] [PubMed]
- Jribi, S.; Saha, B.B.; Koyama, S.; Chakraborty, A.; Ng, K.C. Study on activated carbon/HFO-1234ze(E) based adsorption cooling cycle. Appl. Therm. Eng. 2013, 50, 1570–1575. [Google Scholar] [CrossRef]
- Bo, W.; Jian, L. Advances in synthesis of 1,1,1,3-tetrafluoropropene. Ind. Catal. 2008, 1, 18–22. [Google Scholar]
- Yang, L.; da Rocha, S.R.P. Understanding Solvation in the Low Global Warming Hydrofluoroolefin HFO-1234ze Propellant. J. Phys. Chem. B 2014, 118, 10675–10687. [Google Scholar] [CrossRef]
- Lim, S.; Kim, M.S.; Choi, J.W.; Kim, H.; Ahn, B.S.; Lee, S.D.; Lee, H.; Kim, C.S.; Suh, D.J.; Ha, J.M.; et al. Catalytic dehydrofluorination of 1,1,1,2,3-pentafluoropropane (HFC-245eb) to 2,3,3,3-tetrafluoropropene (HFO-1234yf) using in-situ fluorinated chromium oxyfluoride catalyst. Catal. Today 2017, 293, 42–48. [Google Scholar] [CrossRef]
- Luo, J.W.; Song, J.D.; Jia, W.Z.; Pu, Z.Y.; Lu, J.Q.; Luo, M.F. Catalytic dehydrofluorination of 1,1,1,3,3-pentafluoropropane to 1,3,3,3-tetrafluoropropene over fluorinated NiO/Cr2O3 catalysts. Appl. Surf. Sci. 2018, 433, 904–913. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, W.X.; Liang, Y.; Wang, Y.J.; Lu, J.Q.; Luo, M.F. Pd/AlF3 Catalysts for Catalytic Dehydrofluorination of 1,1,1,3,3-Pentafluoropropane. Chem. Res. Chin. Univ. 2015, 31, 1003–1006. [Google Scholar] [CrossRef]
- Mao, W.; Bai, Y.B.; Jia, Z.H.; Yang, Z.Q.; Hao, Z.J.; Lu, J. Highly efficient gas-phase dehydrofluorination of 1,1,1,3,3-pentafluoropropane to 1,3,3,3-tetrafluoropropene over mesoporous nano-aluminum fluoride prepared from a polyol mediated sol-gel process. Appl. Catal. A 2018, 564, 147–156. [Google Scholar] [CrossRef]
- Wang, Y.; Song, J.; Liu, Y.; Li, X.; Luo, M. Rh/AlF3 Catalysts for Catalytic Dehydrofluorination of 1,1,1,3,3-Pentafluoropropane to 1,3,3,3-Tetrafluoropropene. Org. Fluorine Ind. 2017, 3, 1–5. [Google Scholar]
- Jia, Z.; Mao, W.; Bai, Y.; Li, C.; Lv, J. Preparation of magnesium-aluminum fluoride catalyst and its catalytic performance in gas-phase dehydrofluorination of 1,1,1,3,3-pentafluoropropane. Mod. Chem. Ind. 2018, 38, 87–90. [Google Scholar]
- Boudewijns, T.; Piccinini, M.; Degraeve, P.; Liebens, A.; De Vos, D. Pathway to Vinyl Chloride Production Via Dehydrochlorination of 1,2-Dichloroethane in Ionic Liquid Media. ACS Catal. 2015, 5, 4043–4047. [Google Scholar] [CrossRef]
- Xu, J.M.; Zhao, X.C.; Wang, A.Q.; Zhang, T. Synthesis of nitrogen-doped ordered mesoporous carbons for catalytic dehydrochlorination of 1,2-dichloroethane. Carbon 2014, 80, 610–616. [Google Scholar] [CrossRef]
- Yang, G.J.; Wei, Y.X.; Xu, S.T.; Chen, J.R.; Li, J.Z.; Li, Z.M.; Yu, J.H.; Xu, R.R. Nanosize-Enhanced Lifetime of SAPO-34 Catalysts in Methanol-to-Olefin Reactions. J. Phys. Chem. C 2013, 117, 8214–8222. [Google Scholar] [CrossRef] [Green Version]
- Han, W.F.; Li, X.J.; Tang, H.D.; Wang, Z.K.; Xi, M.; Li, Y.; Liu, H.Z. Preparation of fluorinated Cr2O3 hexagonal prism and catalytic performance for the dehydrofluorination of 1,1-difluoroethane to vinyl fluoride. J. Nanopart. Res. 2015, 17, 12. [Google Scholar] [CrossRef]
- Jia, X.Q.; Quan, H.D.; Tamura, M.; Sekiya, A. Synthesis of microporous fluorinated chromia with a sharp pore distribution. RSC Adv. 2012, 2, 6695–6700. [Google Scholar] [CrossRef]
- Zhang, W.X.; Liang, Y.; Luo, J.W.; Jia, A.P.; Wang, Y.J.; Lu, J.Q.; Luo, M.F. Morphological effects of ordered Cr2O3 nanorods and Cr2O3 nanoparticles on fluorination of 2-chloro-1,1,1-trifluoroethane. J. Mater. Sci. 2016, 51, 6488–6496. [Google Scholar] [CrossRef]
- Sun, H.M.; Wang, L.M.; Chu, D.Q.; Ma, Z.C.; Wang, A.X. Synthesis of porous Cr2O3 hollow microspheres via a facile template-free approach. Mater. Lett. 2015, 140, 35–38. [Google Scholar] [CrossRef]
- Roy, M.; Ghosh, S.; Naskar, M.K. Solvothermal synthesis of Cr2O3 nanocubes via template-free route. Mater. Chem. Phys. 2015, 159, 101–106. [Google Scholar] [CrossRef]
- Han, W.F.; Wang, Z.K.; Li, X.J.; Tang, H.D.; Xi, M.; Li, Y.; Liu, H.Z. Solution combustion synthesis of nano-chromia as catalyst for the dehydrofluorination of 1,1-difluoroethane. J. Mater. Sci. 2016, 51, 11002–11013. [Google Scholar] [CrossRef]
- Meenambika, R.; Ramalingom, S.; Thanu, T.C. Effect of calcinations temperature on the structure of Cr2O3 nanoparticles prepared by novel solvent free synthesis. In Proceedings of the 2013 International Conference on Advanced Nanomaterials and Emerging Engineering Technologies, Chennai, India, 24–26 July 2013; pp. 324–327. [Google Scholar]
- Mukasyan, A.S.; Epstein, P.; Dinka, P. Solution combustion synthesis of nanomaterials. P. Combust. Inst. 2007, 31, 1789–1795. [Google Scholar] [CrossRef]
- Mukasyan, A.S.; Dinka, P. Novel approaches to solution-combustion synthesis of nanomaterials. Int. J. Self-Propag. High-Temp Synth. 2007, 16, 23–35. [Google Scholar] [CrossRef]
- Lima, M.D.; Bonadimann, R.; de Andrade, M.J.; Toniolo, J.C.; Bergmann, C.P. Nanocrystalline Cr2O3 and amorphous CrO3 produced by solution combustion synthesis. J. Eur. Ceram. Soc. 2006, 26, 1213–1220. [Google Scholar] [CrossRef]
- Albonetti, S.; Forni, L.; Cuzzato, P.; Alberani, P.; Zappoli, S.; Trifiro, F. Aging investigation on catalysts for hydrofluorocarbons synthesis. Appl. Catal. A 2007, 326, 48–54. [Google Scholar] [CrossRef]
- Brunet, S.; Boussand, B.; Martin, D. Properties of chromium (III) oxides involved in the catalytic gas phase fluorination of CF3CH2Cl. J. Catal. 1997, 171, 287–292. [Google Scholar] [CrossRef]
- Chung, Y.S.; Lee, H.; Jeong, H.D.; Kim, Y.K.; Lee, H.G.; Kim, H.S.; Kim, S. Enhanced catalytic activity of air-calcined fluorination catalyst. J. Catal. 1998, 175, 220–225. [Google Scholar] [CrossRef]
- Patterson, A.L. The Scherrer Formula for X-Ray Particle Size Determination. Phys. Re. 1939, 56, 978–982. [Google Scholar] [CrossRef]
- Kapteijn, F.; Moulijn, J.A.; Weitkamp, J.; Dalmon, J.A. Handbook of Heterogeneous Catalysis; VCH: Weinheim, Germany, 2008; pp. 290–291. [Google Scholar]
- Lin, Y.; Cai, W.; Tian, X.; Liu, X.; Wang, G.; Liang, C. Polyacrylonitrile/ferrous chloride composite porous nanofibers and their strong Cr-removal performance. J. Mater. Chem. 2011, 21, 991–997. [Google Scholar] [CrossRef]
- Liu, B.; Terano, M. Investigation of the physico-chemical state and aggregation mechanism of surface Cr species on a Phillips CrOx/SiO2 catalyst by XPS and EPMA. J. Mol. Catal. A Chem. 2001, 172, 227–240. [Google Scholar] [CrossRef]
- Gao, S.J.; Dong, C.F.; Luo, H.; Xiao, K.; Pan, X.M.; Li, X.G. Scanning electrochemical microscopy study on the electrochemical behavior of CrN film formed on 304 stainless steel by magnetron sputtering. Electrochim. Acta 2013, 114, 233–241. [Google Scholar] [CrossRef]
- Fu, X.Z.; Luo, X.X.; Luo, J.L.; Chuang, K.T.; Sanger, A.R.; Krzywicki, A. Ethane dehydrogenation over nano-Cr2O3 anode catalyst in proton ceramic fuel cell reactors to co-produce ethylene and electricity. J. Power Sources 2011, 196, 1036–1041. [Google Scholar] [CrossRef]
- Teinz, K.; Wuttke, S.; Borno, F.; Eicher, J.; Kemnitz, E. Highly selective metal fluoride catalysts for the dehydrohalogenation of 3-chloro-1,1,1,3-tetrafluorobutane. J. Catal. 2011, 282, 175–182. [Google Scholar] [CrossRef]
- Navarro, R.M.; Alvarez-Galvan, M.C.; Sanchez-Sanchez, M.C.; Rosa, F.; Fierro, J.L.G. Production of hydrogen by oxidative reforming of ethanol over Pt catalysts supported on Al2O3 modified with Ce and La. Appl. Catal. B 2005, 55, 229–241. [Google Scholar] [CrossRef]
- Ni, J.; Chen, L.; Lin, J.; Kawi, S. Carbon deposition on borated alumina supported nano-sized Ni catalysts for dry reforming of CH4. Nano Energy 2012, 1, 674–686. [Google Scholar] [CrossRef]
- Han, W.F.; Zhang, C.P.; Wang, H.L.; Zhou, S.L.; Tang, H.D.; Yang, L.T.; Wang, Z.K. Sub-nano MgF2 embedded in carbon nanofibers and electrospun MgF2 nanofibers by one-step electrospinning as highly efficient catalysts for 1,1,1-trifluoroethane dehydrofluorination. Catal. Sci. Technol. 2017, 7, 6000–6012. [Google Scholar] [CrossRef]
Sample Availability: Samples of the Cr2O3 catalysts are available from the authors. |







| Samples | Crystal Size (nm) | |||||||
|---|---|---|---|---|---|---|---|---|
| (012) | (104) | (110) | (113) | (024) | (116) | (214) | (300) | |
| Cr2O3-SCS-fresh | 20.8 | 15.8 | 24.4 | 20.5 | 18.1 | 15.1 | 18.2 | 20.7 |
| Cr2O3-SCS-spent | 20.7 | 15.8 | 26.4 | 23.0 | 19.9 | 16.1 | 21.4 | 25.2 |
| Cr2O3-P-fresh | 18.1 | 18.5 | 27.8 | 34.4 | 16.6 | 26.3 | 14.1 | 20.1 |
| Cr2O3-P-spent | 22.4 | 24.3 | 38.3 | 38.1 | 20.4 | 35.5 | 22.7 | 34.9 |
| Cr2O3-C-fresh | >100 | |||||||
| Cr2O3-C-spent | >100 | |||||||
| Samples | Surface Area (m2/g) | Pore Volume (cm3/g) | Average Pore Diameter (nm) |
|---|---|---|---|
| Cr2O3-SCS | 58.2 | 0.3 | 17.2 |
| Cr2O3-P | 33.5 | 0.1 | 16.6 |
| Cr2O3-C | 0.6 | - | - |
| Catalysts | Chromium Oxides, mol% | ||
|---|---|---|---|
| Cr(OH)3 | Cr2O3 | CrO3 | |
| Cr2O3-SCS | 26.2 | 52.1 | 21.7 |
| Cr2O3-C | 6.9 | 77.3 | 15.8 |
| Catalysts | Weight/% | |||
|---|---|---|---|---|
| C | O | F | Cr | |
| Cr2O3-SCS-fresh | 16 | 23.6 | 0 | 60.4 |
| Cr2O3-SCS-spent | 9.5 | 17.9 | 8.0 | 64.6 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Han, W.; Li, X.; Liu, B.; Tang, H.; Li, Y. Solution Combustion Synthesis of Cr2O3 Nanoparticles and the Catalytic Performance for Dehydrofluorination of 1,1,1,3,3-Pentafluoropropane to 1,3,3,3-Tetrafluoropropene. Molecules 2019, 24, 361. https://doi.org/10.3390/molecules24020361
Wang H, Han W, Li X, Liu B, Tang H, Li Y. Solution Combustion Synthesis of Cr2O3 Nanoparticles and the Catalytic Performance for Dehydrofluorination of 1,1,1,3,3-Pentafluoropropane to 1,3,3,3-Tetrafluoropropene. Molecules. 2019; 24(2):361. https://doi.org/10.3390/molecules24020361
Chicago/Turabian StyleWang, Haili, Wenfeng Han, Xiliang Li, Bing Liu, Haodong Tang, and Ying Li. 2019. "Solution Combustion Synthesis of Cr2O3 Nanoparticles and the Catalytic Performance for Dehydrofluorination of 1,1,1,3,3-Pentafluoropropane to 1,3,3,3-Tetrafluoropropene" Molecules 24, no. 2: 361. https://doi.org/10.3390/molecules24020361