Identification and Antioxidant Activity of Flavonoids Extracted from Xinjiang Jujube (Ziziphus jujube Mill.) Leaves with Ultra-High Pressure Extraction Technology
Abstract
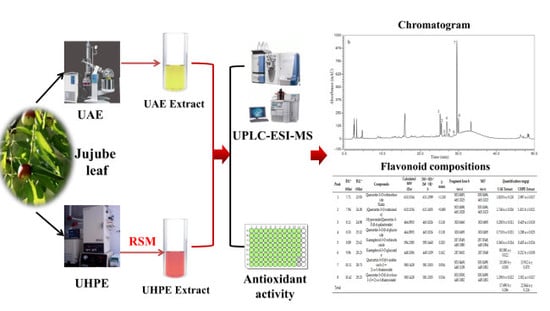
:1. Introduction
2. Materials and Methods
2.1. XJL Sample
2.2. Chemicals and Reagents
2.3. Ultra-High Pressure Extraction (UHPE)
2.4. Ultrasound-Assisted Extraction (UAE)
2.5. Determination of Total Flavonoid Content (TFC)
2.6. HPLC Analysis
2.7. UPLC-ESI-MS Spectrometry Conditions
2.8. Determination of Antioxidant Activities
2.9. Experimental Design
Response Surface Optimization Experiment
2.10. Statistical Analysis
3. Results and Analysis
3.1. Optimization of the UHPE Procedure
3.1.1. Fitting the Model
3.1.2. Model Validation
3.2. Antioxidant Activity
3.3. HPLC Analyses of UAE and UHPE Extracts
3.4. Correlation Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, M.; Gao, Q.H.; Shen, J.; Wang, X.Q.; Ji, X.L. The jujube (Ziziphus jujuba Mill.) fruit: A review of current knowledge of fruit composition and health benefits. J. Agric. Food Chem. 2016, 61, 3351–3363. [Google Scholar]
- Hu, D.; Fan, Y.; Tan, Y.; Tian, Y.; Liu, N.; Wang, L.; Zhao, D.; Wang, C.; Wu, A. Metabolic profiling on alternaria toxins and components of xinjiang jujubes incubated with pathogenic alternaria alternata and alternaria tenuissima via orbitrap high resolution mass spectrometry. J. Agric. Food Chem. 2017, 65, 8466–8474. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Chen, J.; Shi, Q.; Li, Z.; Peng, Z.; Zheng, L.; Wang, X.R. Phytochemical analysis of chinese commercial ziziphus, jujube leaf tea using high performance liquid chromatography–electrospray ionization-time of flight mass spectrometry. Food Res. Int. 2014, 56, 47–54. [Google Scholar] [CrossRef]
- Zhang, R.; Chen, J.; Shi, Q.; Li, Z.; Peng, Z.; Zheng, L.; Wang, X. Quality control method for commercially available wild jujube leaf tea based on HPLC characteristic fingerprint analysis of flavonoid compounds. J. Sep. Sci. 2014, 37, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, R.T.; Chopda, M.Z. Phyto-pharmacology of Ziziphus jujuba Mill—A plant review. Pharm. Rev. 2009, 3, 320–329. [Google Scholar]
- Damiano, S.; Forino, M.; De, A.; Vitali, L.A.; Lupidi, G.; Taglialatela-Scafati, O. Antioxidant and antibiofilm activities of secondary metabolites from Ziziphus jujuba leaves used for infusion preparation. Food Chem. 2017, 230, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Cui, X.; Cheng, N.; Cao, W.; Wu, Y.; Guo, S.; Zhang, L.; Ho, C.T.; Bai, N. Hepatoprotective standardized EtOH-water extract of the leaves of Ziziphus jujuba. Food Funct. 2017, 8, 816–822. [Google Scholar] [CrossRef]
- Hovaneţ, M.-V.; Oprea, E.; Ancuceanu, R.V.; Duţu, L.E.; Budura, E.A.; Şeremet, O.; Ancu, I.; Moroşan, E. Wound healing properties of Ziziphus jujuba Mill. leaves. Rom. Biotech. Lett. 2016, 21, 11842–11849. [Google Scholar]
- Liu, H.X.; Xu, M.Q.; Li, S.P.; Tian, S.; Guo, M.X.; Qi, J.Y.; He, C.J.; Zhao, X.S. Jujube leaf green tea extracts inhibits hepatocellular carcinoma cells by activating AMPK. Oncotarget 2017, 8, 110566–110575. [Google Scholar] [CrossRef]
- Jo, Y.; Lim, S.; Chang, P.S.; Choi, Y.J. The possible presence of natural β-d-glucosidase inhibitors in jujube leaf extract. Food Chem. 2016, 194, 212–217. [Google Scholar] [CrossRef]
- Ghasemzadeh, A.; Ghasemzadeh, N. Flavonoids and phenolic acids: Role and biochemical activity in plants and human. J. Med. Plant. Res. 2011, 5, 6697–6703. [Google Scholar] [CrossRef]
- García-Villalba, R.; Espín, J.C.; Tomás-Barberán, F.A.; Rocha-Guzmán, N.E. Comprehensive characterization by LC-DAD-MS/MS of the phenolic composition of seven Quercus leaf teas. J. Food Compos. Anal. 2017, 63, 38–46. [Google Scholar] [CrossRef]
- Guo, S.; Duan, J.A.; Tang, Y.P.; Qian, Y.F.; Zhao, J.L.; Qian, D.W. Simultaneous qualitative and quantitative analysis of triterpenic acids, saponins and flavonoids in the leaves of two Ziziphus species by HPLC–PDA–MS/ELSD. J. Pharm. Biomed. Anal. 2011, 56, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Pei, X.P.; Du, C.H.; Yan, Y.; Shen, J.X.; Pei, M.R.; Bai, Y. Determination of rutin from leaves of Ziziphus jujuba gathered in different areas and different periods by HPLC–UV. Chin. J. Exp. Tradit. Med. Formul. 2011, 17, 94–98. [Google Scholar]
- Mustafa, A.; Turner, C. Pressurized liquid extraction as a green approach in food and herbal plants extraction: A review. Anal. Chim. Acta 2011, 703, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Singh, A.K.; Keshari, A.K.; Raj, V.; Rai, A.; Maity, S. Modern extraction techniques for drugs and medicinal agents. Ingred. Extr. Phys.-Chem. Methods Food 2017, 65–106. [Google Scholar] [CrossRef]
- You, Q.H.; Yin, X.L.; Zhang, S.N.; Jiang, Z.H. Extraction, purification, and antioxidant activities of polysaccharides from Tricholoma mongolicum Imai. Carbohydr. Polym. 2014, 99, 1–10. [Google Scholar]
- Bimakr, M.; Rahman, R.A.; Taip, F.S.; Ganjloo, A.; Salleh, L.M.; Selamat, J.; Hmid, A.; Zaidul, I.S.M. Comparison of different extraction methods for the extraction of major bioactive flavonoid compounds from spearmint (Mentha spicata L.) leaves. Food Bioprod. Process 2011, 89, 67–72. [Google Scholar] [CrossRef]
- Yu, L.; Xu, X.Q.; Xue, C.H.; Chang, Y.G.; Ge, L.; Wang, Y.C.; Zhang, C.Y.; Liu, G.C.; He, C. Enzymatic preparation and structural determination of oligosaccharides derived from sea cucumber (Acaudina molpadioides) fucoidan. Food Chem. 2013, 139, 702–709. [Google Scholar] [CrossRef]
- Huang, H.W.; Hsu, C.P.; Yang, B.B.; Wang, C.Y. Advances in the extraction of natural ingredients by high pressure extraction technology. Trends Food Sci. Tech. 2013, 33, 54–62. [Google Scholar] [CrossRef]
- Xi, J.; Yan, L. Optimization of pressure-enhanced solid-liquid extraction of flavonoids from flos sophorae, and evaluation of their antioxidant activity. Sep. Purif. Tech. 2017, 175, 170–176. [Google Scholar] [CrossRef]
- Sarikurkcu, C.; Tepe, B.; Kocak, M.S.; Uren, M.C. Metal concentration and antioxidant activity of edible mushrooms from turkey. Food Chem. 2015, 175, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Gan, C.Y.; Latiff, A.A. Optimisation of the solvent extraction of bioactive compounds from Parkia speciosa pod using response surface methodology. Food Chem. 2011, 124, 1277–1283. [Google Scholar] [CrossRef]
- Duba, K.S.; Fiori, L. Extraction of bioactives from food processing residues using techniques performed at high pressures. Curr. Opin. Food Sci. 2015, 5, 14–22. [Google Scholar] [CrossRef]
- Michiels, J.A.; Kevers, C.; Pincemail, J.; Defraigne, J.O.; Dommes, J. Extraction conditions can greatly influence antioxidant capacity assays in plant food matrices. Food Chem. 2012, 130, 986–993. [Google Scholar] [CrossRef]
- Chen, R.Z.; Jin, C.G.; Li, H.P.; Liu, Z.Q.; Lu, J.; Li, S.Z.; Yang, S.M. Ultrahigh pressure extraction of polysaccharides from cordyceps militaris, and evaluation of antioxidant activity. Sep. Purif. Tech. 2014, 134, 90–99. [Google Scholar] [CrossRef]
- Corrales, M.; Toepfl, S.; Butz, P.; Knorr, D.; Tauscher, B. Extraction of anthocyanins from grape by-products assisted by ultrasonics, high hydrostatic pressure or pulsed electric fields: A comparison. Innov. Food Sci. Emerg. Tech. 2008, 9, 85–91. [Google Scholar] [CrossRef]
- Brioneslabarca, V. High hydrostatic pressure and ultrasound extractions of antioxidant compounds, sulforaphane and fatty acids from chilean papaya (vasconcellea pubescens) seeds: Effects of extraction conditions and methods. LWT Food Sci. Tech. 2015, 60, 525–534. [Google Scholar] [CrossRef]
- Zhang, S.; Xi, J.; Wang, C. High hydrostatic pressure extraction of flavonoids from propolis. J. Chem. Tech. Biotech. 2005, 80, 50–54. [Google Scholar]
- Corrales, M.; García, A.F.; Butz, P.; Tauscher, B. Extraction of anthocyanins from grape skins assisted by high hydrostatic pressure. J. Food Eng. 2009, 90, 415–421. [Google Scholar] [CrossRef]
- Sadus, R.J. High pressure phase behaviour of multicomponent fluid mixtures. High Press. Phase Behav. Multicompon. Fluid Mixtures 1992, 95, xv–xvii. [Google Scholar]
- Romero-Díez, R.; Rodríguez-Rojo, S.; Cocero, M.J.; Duarte, C.M.M.; Matias, A.A.; Bronze, M.R. Phenolic characterization of aging wine lees: Correlation with antioxidant activities. Food Chem. 2018, 259, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Pandey, G.; Khatoon, S.; Pandey, M.M.; Aks, R. Altitudinal variation of berberine, total phenolics and flavonoid content in thalictrum foliolosum and their correlation with antimicrobial and antioxidant activities. J. Ayurveda Integr. Med. 2018, 9, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.F.; Hu, Z.Q.; Yu, Y.H.; Mou, R.X.; Zhu, Z.W.; Beta, T. Phenolic acids, anthocyanins, proanthocyanidins, antioxidant activity, minerals and their correlations in non-pigmented, red, and black rice. Food Chem. 2018, 239, 733–741. [Google Scholar] [CrossRef] [PubMed]
- Kallithraka, S.; Mohdaly, A.A.A.; Makris, D.P.; Kefalas, P. Determination of major anthocyanin pigments in Hellenic native grape varieties (Vitis vinifera sp.): Association with antiradical activity. J. Food Compos. Anal. 2005, 18, 375–386. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds 1–8 are available from the authors. |




| Code | X1: Temperature (°C) | X2: Pressure (MPa) | X3: Time (min) | X4: Liquid-to-Solid Ratio (mL/g) |
|---|---|---|---|---|
| −1 | 35 | 200 | 8 | 30:1 |
| 0 | 45 | 300 | 10 | 40:1 |
| +1 | 55 | 400 | 12 | 50:1 |
| Runs | X1: Temperature (°C) | X2: Pressure (MPa) | X3: Time (min) | X4: Liquid-to-Solid Ratio (V:m) | Y: Flavonoid Yield (mg/g) |
|---|---|---|---|---|---|
| 1 | 45 | 300 | 10 | 40 | 25.14 |
| 2 | 55 | 200 | 10 | 40 | 23.69 |
| 3 | 55 | 300 | 10 | 50 | 25.04 |
| 4 | 45 | 200 | 12 | 40 | 23.83 |
| 5 | 35 | 300 | 10 | 50 | 23.19 |
| 6 | 55 | 300 | 10 | 30 | 24.43 |
| 7 | 45 | 300 | 10 | 40 | 25.03 |
| 8 | 45 | 300 | 10 | 40 | 24.99 |
| 9 | 55 | 400 | 10 | 40 | 24.91 |
| 10 | 55 | 300 | 12 | 40 | 25.44 |
| 11 | 55 | 300 | 8 | 40 | 23.05 |
| 12 | 45 | 300 | 12 | 30 | 24.59 |
| 13 | 45 | 200 | 8 | 40 | 21.74 |
| 14 | 35 | 300 | 10 | 30 | 22.76 |
| 15 | 45 | 400 | 10 | 50 | 24.78 |
| 16 | 35 | 400 | 10 | 40 | 23.52 |
| 17 | 35 | 300 | 8 | 40 | 21.59 |
| 18 | 45 | 300 | 10 | 40 | 25.14 |
| 19 | 45 | 300 | 10 | 40 | 25.19 |
| 20 | 45 | 200 | 10 | 50 | 23.26 |
| 21 | 45 | 400 | 8 | 40 | 22.92 |
| 22 | 45 | 300 | 8 | 50 | 22.59 |
| 23 | 45 | 400 | 10 | 30 | 24.48 |
| 24 | 45 | 300 | 12 | 50 | 25.29 |
| 25 | 45 | 300 | 8 | 30 | 22.66 |
| 26 | 35 | 300 | 12 | 40 | 23.75 |
| 27 | 35 | 200 | 10 | 40 | 21.46 |
| 28 | 45 | 200 | 10 | 30 | 22.73 |
| 29 | 45 | 400 | 12 | 40 | 25.37 |
| Source | Sum of Squares | df | Mean Square | F Value | p-Value Prob > F |
|---|---|---|---|---|---|
| Model | 42.38 | 14 | 3.03 | 276.25 | <0.0001 |
| X1: temperature | 8.82 | 1 | 8.82 | 805.18 | <0.0001 |
| X2: pressure | 7.16 | 1 | 7.16 | 653.46 | <0.0001 |
| X3: time | 15.69 | 1 | 15.69 | 1431.42 | <0.0001 |
| X4: Liquid–material ratio | 0.52 | 1 | 0.52 | 47.53 | <0.0001 |
| X1X2 | 0.18 | 1 | 0.18 | 16.1 | 0.0013 |
| X1X3 | 0.013 | 1 | 0.013 | 1.21 | 0.2905 |
| X1X4 | 8.10 × 10−3 | 1 | 8.10 × 10−3 | 0.74 | 0.4044 |
| X2X3 | 0.032 | 1 | 0.032 | 2.96 | 0.1076 |
| X2X4 | 0.013 | 1 | 0.013 | 1.21 | 0.2905 |
| X3X4 | 0.15 | 1 | 0.15 | 13.53 | 0.0025 |
| X12 | 4.4 | 1 | 4.4 | 401.08 | <0.0001 |
| X22 | 4.58 | 1 | 4.58 | 418.31 | <0.0001 |
| X32 | 4.41 | 1 | 4.41 | 402.29 | <0.0001 |
| X42 | 1.32 | 1 | 1.32 | 120.88 | <0.0001 |
| Residual | 0.15 | 14 | 0.011 | ||
| Lack of fit | 0.13 | 10 | 0.013 | 1.77 | 0.3062 |
| Pure error | 0.028 | 4 | 7.07 × 10−3 | ||
| Cor total | 42.54 | 28 | |||
| R2 | 0.9964 | ||||
| Adj R2 | 0.9928 | ||||
| Pred R2 | 0.9820 | ||||
| Adeq precision | 53.151 |
| Peak | Rt1 a | Rt2 a | Compounds | Calculated MW | [M + H]+/[M − H]− b | Δ mmu | Fragment Ions b | MS2 | Quantification (mg/g) | Ref. | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| (Min) | (Min) | (Da) | (m/z) | (m/z) | UAE Extract | UHPE Extract | |||||
| 1 | 7.71 | 23.93 | Quercetin-3-O-robinobioside | 610.1534 | 611.1599 | −1.230 | 303.0495; 465.1025 | 303.0496; 465.1023 | 1.820 b ± 0.120 | 2.987 a ± 0.017 | [4] |
| 2 | 7.94 | 24.38 | Rutin (Quercetin-3-O-rutinoside) | 610.1534 | 611.1605 | −0.680 | 303.0498; 465.1028 | 303.0496; 465.1023 | 2.740 a ± 0.026 | 1.631 b ± 0.021 | [4] |
| 3 | 8.11 | 24.98 | Hyperoside(Quercetin-3-O-β-d-galactoside) | 464.0955 | 465.1026 | 0.118 | 303.0460 | 303.0495 | 0.200 b ± 0.011 | 0.419 a ± 0.010 | [4] |
| 4 | 8.33 | 25.32 | Quercetin-3-O-β-d-glucoside | 464.0955 | 465.1026 | 0.118 | 303.0495 | 303.0495 | 0.710 b ± 0.021 | 1.208 a ± 0.025 | [4] |
| 5 | 8.89 | 25.62 | Kaempferol-3-O-robinobioside | 594.1585 | 595.1660 | 0.203 | 287.0549; 449.1080 | 287.0548; 449.1084 | 0.360 a ± 0.014 | 0.403 a ± 0.016 | [4] |
| 6 | 9.96 | 28.23 | Kaempferol-3-O-glucoside | 448.1006 | 449.1109 | 0.162 | 287.0602 | 287.0548 | 00.390 a ± 0.022 | 0.232 b ± 0.030 | [4] |
| 7 | 10.11 | 28.73 | Quercetin-3-O-β-l-arabinosyl-(1→2)-α-l-rhamnoside | 580.1428 | 581.1503 | 0.054 | 303.0469; 449.1109 | 303.0499; 449.1081 | 10.180 b ± 0.050 | 13.912 a ± 0.070 | [4] |
| 8 | 10.42 | 29.25 | Quercetin-3-O-β-d-xylosyl-(1→2)-α-l-rhamnoside | 580.1428 | 581.1505 | 0.534 | 303.0500; 449.1082 | 303.0499; 449.1081 | 1.290 b ± 0.022 | 2.052 a ± 0.027 | [4] |
| Total | 17.690 b ± 0.286 | 22.844 a ± 0.226 | [4] | ||||||||
| DPPH | ABTS | TFC | Qr | R | Qa | Qu | Kr | A | Ql | Qx | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| DPPH | - | 0.996 ** | 0.909 ** | 0.888 ** | 0.637 * | 0.908 ** | 0.617 * | 0.842 ** | 0.609 * | 0.915 ** | 0.907 ** |
| ABTS | 0.996 ** | - | 0.909 ** | 0.894 ** | 0.666 ** | 0.904 ** | 0.579 * | 0.855 ** | 0.639 * | 0.910 ** | 0.896 ** |
| TFC | 0.909 ** | 0.909 ** | - | 0.993 ** | 0.779 ** | 0.990 ** | 0.583 * | 0.962 ** | 0.745 ** | 0.992 ** | 0.971 ** |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Liu, P.; Li, L.; Huang, Y.; Pu, Y.; Hou, X.; Song, L. Identification and Antioxidant Activity of Flavonoids Extracted from Xinjiang Jujube (Ziziphus jujube Mill.) Leaves with Ultra-High Pressure Extraction Technology. Molecules 2019, 24, 122. https://doi.org/10.3390/molecules24010122
Zhang L, Liu P, Li L, Huang Y, Pu Y, Hou X, Song L. Identification and Antioxidant Activity of Flavonoids Extracted from Xinjiang Jujube (Ziziphus jujube Mill.) Leaves with Ultra-High Pressure Extraction Technology. Molecules. 2019; 24(1):122. https://doi.org/10.3390/molecules24010122
Chicago/Turabian StyleZhang, Li, Pengzhan Liu, Linlin Li, Ying Huang, Yunfeng Pu, Xujie Hou, and Lijun Song. 2019. "Identification and Antioxidant Activity of Flavonoids Extracted from Xinjiang Jujube (Ziziphus jujube Mill.) Leaves with Ultra-High Pressure Extraction Technology" Molecules 24, no. 1: 122. https://doi.org/10.3390/molecules24010122