Associated-Extraction Efficiency of Six Cyclodextrins on Various Flavonoids in Puerariae Lobatae Radix
Abstract
:1. Introduction
2. Experiment
2.1. Chinese Herbal Medicines and Reagents
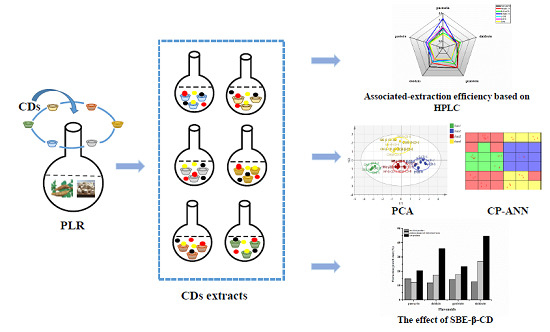
2.2. Preparation of Samples
2.3. HPLC Analysis
2.4. Statistical Analysis
2.4.1. Evaluation of Associated-Extraction Efficiency Using HPLC Analysis
2.4.2. Principal Component Analysis (PCA)
2.4.3. Counter-Propagation Artificial Neural Network (CP-ANN)
3. Results and Discussion
3.1. Evaluation of Associated-Extraction Efficiency Using HPLC Analysis
3.1.1. Associated-Extraction Efficiency of Five PRL Flavonoids by Six CDs
3.1.2. Principal Component Analysis (PCA)
3.1.3. Counter-Propagation Artificial Neural Networks (CP-ANN)
3.2. Associated-Extraction Efficiency of SBE-β-CD for Flavonoids from Different Processed Products of PLR
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wong, K.H.; Razmovski-Naumovski, V.; Li, K.M.; Li, G.Q.; Chan, K. Differentiating Puerariae Lobatae Radix and Puerariae Thomsonii Radix using HPTLC coupled with multivariate classification analyses. J. Pharm. Biomed. Anal. 2014, 95, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Lam, T.N.; Zuo, Z. Radix Puerariae: An overview of its chemistry, pharmacology, pharmacokinetics, and clinical use. J. Clin. Pharmacol. 2013, 53, 787–811. [Google Scholar] [CrossRef] [PubMed]
- Ling, X.; Xiang, Y.; Tang, Q.; Jin, Z.; Chen, F.; Tan, X. Soybean Milk Inhibits Absorption and Intestinal Transmembrane Transport of Gegen in Rats. Evid.-Based Complement. Altern. Med. 2017, 2017, 7146813. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.H.; Razmovski-Naumovski, V.; Li, K.M.; Li, G.Q.; Chan, K. Comparing morphological, chemical and anti-diabetic characteristics of Puerariae Lobatae Radix and Puerariae Thomsonii Radix. J. Ethnopharmacol. 2015, 164, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Gao, Y.; Ding, H.; Liu, S.; Han, X.; Gui, J.; Liu, D. Subcritical ethanol extraction of flavonoids from Moringa oleifera leaf and evaluation of antioxidant activity. Food Chem. 2017, 218, 152–158. [Google Scholar] [CrossRef]
- Huang, H.; Liaw, E. Extraction Optimization of Flavonoids from Hypericum formosanum and Matrix Metalloproteinase-1 Inhibitory Activity. Molecules 2017, 22, 2172. [Google Scholar] [CrossRef]
- Ciulu, M.; Cádiz-Gurrea, M.; Segura-Carretero, A. Extraction and Analysis of Phenolic Compounds in Rice: A Review. Molecules 2018, 23, 2890. [Google Scholar] [CrossRef]
- Bai, C.; Tian, B.; Zhao, T.; Huang, Q.; Wang, Z. Cyclodextrin-Catalyzed Organic Synthesis: Reactions, Mechanisms, and Applications. Molecules 2017, 22, 1475. [Google Scholar] [CrossRef]
- Jansook, P.; Ogawa, N.; Loftsson, T. Cyclodextrins: Structure, physicochemical properties and pharmaceutical applications. Int. J. Pharm. 2018, 535, 272–284. [Google Scholar] [CrossRef]
- Irie, T.; Uekama, K. Pharmaceutical applications of cyclodextrins. III. Toxicological issues and safety evaluation. J. Pharm. Sci. 1997, 86, 147–162. [Google Scholar] [CrossRef]
- Muankaew, C.; Loftsson, T. Cyclodextrin-Based Formulations: A Non-Invasive Platform for Targeted Drug Delivery. Basic Clin. Pharmacol. 2018, 122, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, Q.S.; Cui, Y.L.; Meng, F.C.; Lin, K.M. Changes in the intestinal absorption mechanism of icariin in the nanocavities of cyclodextrins. Int. J. Nanomed. 2012, 7, 4239–4249. [Google Scholar]
- Xie, J.; Xu, Y.; Shishir, M.R.I.; Zheng, X.; Chen, W. Green extraction of mulberry anthocyanin with improved stability using β-cyclodextrin. J. Sci. Food Agrc. 2018. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Liu, Y.; Liu, T.; Yuan, Y.; Yue, T.; Cai, R.; Wang, Z. Extraction of Epigallocatechin Gallate and Epicatechin Gallate from Tea Leaves Using β-Cyclodextrin. J. Food Sci. 2017, 82, 394–400. [Google Scholar] [CrossRef] [PubMed]
- Ratnasooriya, C.C.; Rupasinghe, H.P.V. Extraction of phenolic compounds from grapes and their pomace using β-cyclodextrin. Food Chem. 2012, 134, 625–631. [Google Scholar] [CrossRef] [PubMed]
- Parmar, I.; Sharma, S.; Rupasinghe, H.P.V. Optimization of β-cyclodextrin-based flavonol extraction from apple pomace using response surface methodology. J. Food Sci. Technol. 2015, 52, 2202–2210. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Yang, J.; Wang, M.; Zhang, H.; Liu, Y.; Ren, X.; Qi, A. Combination of counterpropagation artificial neural networks and antioxidant activities for comprehensive evaluation of associated-extraction efficiency of various cyclodextrins in the traditional Chinese formula Xue-Zhi-Ning. J. Pharm. Biomed. 2015, 115, 580–586. [Google Scholar] [CrossRef]
- You, G.; Sun, L.; Cao, X.; Li, H.; Wang, M.; Liu, Y.; Ren, X. Comprehensive evaluation of solubilization of flavonoids by various cyclodextrins using high performance liquid chromatography and chemometry. LWT 2018, 94, 172–177. [Google Scholar] [CrossRef]
- Ghodadra, A.; Alhilali, L.; Fakhran, S. Principal Component Analysis of Diffusion Tensor Images to Determine White Matter Injury Patterns Underlying Postconcussive Headache. Am. J. Neuroradiol. 2016, 37, 274–278. [Google Scholar] [CrossRef]
- Zhuang, H.; Ni, Y.; Kokot, S. Combining HPLC-DAD and ICP-MS data for improved analysis of complex samples: Classification of the root samples from Cortex moutan. Chemom. Intell. Lab. Syst. 2014, 135, 183–191. [Google Scholar] [CrossRef]
- Konishi, T. Principal component analysis for designed experiments. BMC Bioinform. 2015, 16. [Google Scholar] [CrossRef] [PubMed]
- Ni, Y.; Mei, M.; Kokot, S. One- and two-dimensional gas chromatography–mass spectrometry and high performance liquid chromatography–diode-array detector fingerprints of complex substances: A comparison of classification performance of similar, complex Rhizoma Curcumae samples with the aid of chemometrics. Anal. Chim. Acta 2012, 712, 37–44. [Google Scholar] [PubMed]
- Xu, Y.; Zhao, Y.; Guo, X.; Li, Y.; Zhang, Y. Plasma metabolic profiling analysis of neurotoxicity induced by oxaliplatin using metabonomics and multivariate data analysis. Toxicol. Res. UK 2018, 7, 529–537. [Google Scholar] [CrossRef] [PubMed]
- Ballabio, D.; Consonni, V.; Todeschini, R. The Kohonen and CP-ANN toolbox: A collection of MATLAB modules for Self Organizing Maps and Counterpropagation Artificial Neural Networks. Chemom. Intell. Lab. Syst. 2009, 98, 115–122. [Google Scholar] [CrossRef]
- Ballabio, D.; Vasighi, M. A MATLAB toolbox for Self Organizing Maps and supervised neural network learning strategies. Chemom. Intell. Lab. Syst. 2012, 118, 24–32. [Google Scholar] [CrossRef]
- Erić, S.; Kalinić, M.; Popović, A.; Zloh, M.; Kuzmanovski, I. Prediction of aqueous solubility of drug-like molecules using a novel algorithm for automatic adjustment of relative importance of descriptors implemented in counter-propagation artificial neural networks. Int. J. Pharm. 2012, 437, 232–241. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Not available. |





| Groups | Peak Area | ||||
|---|---|---|---|---|---|
| Puerarin | Daidzein | Genisten | Daidzin | Genistin | |
| sulfobutyl ether β-cyclodextrin | 2,029,213 ± 203,886 ** | 221,198 ± 43,008 | 36188 ± 10293 ** | 305,795 ± 51,497 ** | 648,695 ± 65,923 |
| carboxymethyl-β-cyclodextrin | 1,930,712 ± 247,991 ** | 270,287 ± 28,210 ** | 27,443 ± 6175 | 241,245 ± 46,006 * | 746,623 ± 94,570 * |
| hydroxypropyl-γ-cyclodextrin | 2,179,303 ± 456,406 ** | 227,414 ± 67,279 | 18,593 ± 833 | 172,658 ± 54,210 | 668,197 ± 31,088 |
| hydroxypropyl-β-cyclodextrin | 3,024,023 ± 836,080 ** | 219,235 ± 183,98 | 20,889 ± 3454 | 226,944 ± 12,877 ** | 772,266 ± 238,888 |
| γ-cyclodextrin | 1,915,263 ± 264,897 * | 201,910 ± 46,723 | 22,348 ± 579 | 229,948 ± 58,279 | 669,958 ± 178,231 |
| β-cyclodextrin | 2,113,199 ± 58,178 ** | 206,347 ± 6211 | 21,097 ± 4635 | 189,898 ± 43,431 | 709,756 ± 33,611 * |
| original aqueous extracts | 1,423,886 ± 304,315 | 210,638 ± 33,799 | 20,610 ± 5023 | 183,151 ± 11,279 | 599,673 ± 81,374 |
| Groups | Flavonoids | ||||
|---|---|---|---|---|---|
| Puerarin | Daidzein | Genistein | Daidzin | Genistin | |
| sulfobutyl ether β-cyclodextrin | 42.51 | 5.01 | 75.59 | 66.96 | 8.17 |
| carboxymethyl-β-cyclodextrin | 35.59 | 28.32 | 33.16 | 31.72 | 24.50 |
| hydroxypropyl-γ-cyclodextrin | 10.03 | 31.37 | 40.78 | 4.51 | 7.43 |
| hydroxypropyl-β-cyclodextrin | 112.38 | 4.08 | 1.36 | 23.91 | 28.78 |
| γ-cyclodextrin | 34.51 | −4.14 | 8.43 | 25.55 | 11.72 |
| β-cyclodextrin | 48.41 | −2.04 | 2.36 | 3.68 | 18.36 |
| Groups | Flavonoids | Sum | ||||
|---|---|---|---|---|---|---|
| Puerarin | Daidzein | Genistein | Daidzin | Genistin | ||
| sulfobutyl ether β-cyclodextrin | 1.43 | 1.05 | 1.76 | 1.67 | 1.08 | 6.98 |
| carboxymethyl-β-cyclodextrin | 1.36 | 1.28 | 1.33 | 1.32 | 1.25 | 6.53 |
| hydroxypropyl-γ-cyclodextrin | 1.10 | 1.31 | 1.41 | 1.05 | 1.07 | 5.94 |
| hydroxypropyl-β-cyclodextrin | 2.12 | 1.04 | 1.01 | 1.24 | 1.29 | 6.71 |
| γ-cyclodextrin | 1.35 | 0.96 | 1.08 | 1.26 | 1.12 | 5.76 |
| β-cyclodextrin | 1.48 | 0.98 | 1.02 | 1.04 | 1.18 | 5.71 |
| original aqueous extracts | 1 | 1 | 1 | 1 | 1 | 5 |
| Medicinal Herbs | Flavonoids | Original Aqueous Extracts | Sulfobutyl Ether β-Cyclodextrin |
|---|---|---|---|
| stir-fried product | Puerarin | 4,962,426 ± 123,669 | 5,700,551 ± 297,235 * |
| Daidzin | 1,285,994 ± 47,366 | 1,439,234 ± 11,338 | |
| Genistein | 273,012 ± 3154 | 312,108 ± 8207 * | |
| Daidzein | 200,883 ± 2281 | 226,512 ± 28,244 | |
| product simmered with wheat bran | Puerarin | 4,348,354 ± 33,017 | 4,880,039 ± 158,484 ** |
| Daidzin | 1,330,505 ± 79,428 | 1560,305 ± 32,950 ** | |
| Genistein | 455,096 ± 39,482 | 535,801 ± 8466 * | |
| Daidzein | 325,621 ± 27,850 | 412,680 ± 8902 ** | |
| raw product | Puerarin | 6,678,584 ± 698,346 | 8,045,093 ± 239,842 * |
| Daidzin | 1830,292 ± 61,375 | 2,487,306 ± 150,734 | |
| Genistein | 371,929 ± 2880 | 458,303 ± 7741 ** | |
| Daidzein | 274,732 ± 6077 | 397,165 ± 2822 ** |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, T.; Liu, F.; Sun, L.; Huo, H.; Ren, X.; Wang, M. Associated-Extraction Efficiency of Six Cyclodextrins on Various Flavonoids in Puerariae Lobatae Radix. Molecules 2019, 24, 93. https://doi.org/10.3390/molecules24010093
Feng T, Liu F, Sun L, Huo H, Ren X, Wang M. Associated-Extraction Efficiency of Six Cyclodextrins on Various Flavonoids in Puerariae Lobatae Radix. Molecules. 2019; 24(1):93. https://doi.org/10.3390/molecules24010093
Chicago/Turabian StyleFeng, Tao, Fan Liu, Lili Sun, Hongna Huo, Xiaoliang Ren, and Meng Wang. 2019. "Associated-Extraction Efficiency of Six Cyclodextrins on Various Flavonoids in Puerariae Lobatae Radix" Molecules 24, no. 1: 93. https://doi.org/10.3390/molecules24010093