Direct Experimental Evidence of Biomimetic Surfaces with Chemical Modifications Interfering with Adhesive Protein Adsorption
Abstract
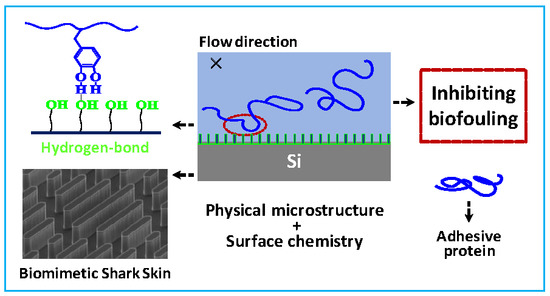
:1. Introduction
2. Results and discussion
2.1. Fabrications and Properties of Functionalized Surfaces
2.2. Interpretation through Direct Force Measurements
2.3. Interpretation through In Situ Adsorption Measurements
3. Materials and Methods
3.1. Preparation of Surface Structures
3.2. Preparation of Surface Chemistries
3.3. Surface Characterization
3.4. AFM Tip Modification and Characterization
3.5. Force-Measuring Technique
3.6. Quartz Crystal Microbalance with Dissipation Monitoring (QCM-D)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sedó, J.; Saizposeu, J.; Busqué, F.; Ruizmolina, D. Catechol-based biomimetic functional materials. Adv. Mater. 2013, 25, 653–701. [Google Scholar] [CrossRef] [PubMed]
- Anderson, T.H.; Yu, J.; Estrada, A.; Hammer, D.M.U.; Waite, P.J.H.; Israelachvili, P.J.N. The contribution of DOPA to substrate-peptide adhesion and internal cohesion of mussel-inspired synthetic peptide films. Adv. Funct. Mater. 2010, 20, 4196–4205. [Google Scholar] [CrossRef] [PubMed]
- Callow, J.A.; Callow, M.E. Trends in the development of environmentally friendly fouling-resistant marine coatings. Nat. Commun. 2011, 2, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Epstein, A.K.; Hong, D.; Kim, P.; Aizenberg, J. Biofilm attachment reduction on bioinspired, dynamic, micro-wrinkling surfaces. New J. Phys. 2013, 15, 095018. [Google Scholar] [CrossRef] [Green Version]
- Banerjee, I.; Pangule, R.C.; Kane, R.S. Antifouling coatings: Recent developments in the design of surfaces that prevent fouling by proteins, bacteria, and marine organisms. Adv. Mater. 2011, 23, 690–718. [Google Scholar] [CrossRef] [PubMed]
- Meyer, B. Approaches to prevention, removal and killing of biofilms. Int. Biodeterior. Biodegr. 2003, 51, 249–253. [Google Scholar] [CrossRef]
- Dalsin, J.L.; Hu, B.H.; Lee, B.P.; Messersmith, P.B. Mussel adhesive protein mimetic polymers for the preparation of nonfouling surfaces. J. Am. Chem. Soc. 2003, 125, 4253–4258. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Yang, H.; Gao, H.; Fu, M.; Huang, S.; Zhang, W.; Hu, G.; Liu, F.; Ma, A.; Sun, K.; et al. Adsorption and orientation of 3,4-dihydroxy-L-phenylalanine onto tunable monolayer films. J. Phys. Chem. C 2017, 121, 11544–11551. [Google Scholar] [CrossRef]
- Nishimoto, S.; Bhushan, B. Bioinspired self-cleaning surfaces with superhydrophobicity, superoleophobicity, and superhydrophilicity. RSC Adv. 2013, 3, 671–690. [Google Scholar] [CrossRef]
- Krishnan, S.; Wang, N.; Ober, C.K.; Finlay, J.A.; Callow, M.E.; Callow, J.A.; Hexemer, A.; Sohn, K.E.; Kramer, E.J.; Fischer, D.A. Comparison of the fouling release properties of hydrophobic fluorinated and hydrophilic PEGylated block copolymer surfaces: Attachment strength of the diatom Navicula and the green alga Ulva. Biomacromolecules 2006, 7, 1449–1462. [Google Scholar] [CrossRef]
- Prime, K.L.; Whitesides, G.M. Self-assembled organic monolayers: Model systems for studying adsorption of proteins at surfaces. Science 1991, 252, 1164–1167. [Google Scholar] [CrossRef] [PubMed]
- Hook, F.; Kasemo, B.; Nylander, T.; Fant, C.; Kristin Sott, A.; Elwing, H. Variations in coupled water, viscoelastic properties, and film thickness of a mefp-1 protein film during adsorption and cross-linking: A quartz crystal microbalance with dissipation monitoring, ellipsometry, and surface plasmon resonance study. Anal. Chem. 2001, 73, 5796–5804. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, J.F.; Carman, M.L.; Estes, T.G.; Feinberg, A.W.; Wilson, L.H.; Callow, M.E.; Callow, J.A.; Finlay, J.A.; Brennan, A.B. Engineered antifouling microtopographies—effect of feature size, geometry, and roughness on settlement of zoospores of the green alga Ulva. Biofouling 2007, 23, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Ralston, E.; Swain, G. Bioinspiration—the solution for biofouling control? Bioinspir. Biomim. 2009, 4, 015007. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Ding, Y.; Ao, Z.; Zhou, Y.; Wang, S.; Jiang, L. Fabricating surfaces with tunable wettability and adhesion by ionic liquids in a wide range. Small 2015, 11, 1782–1786. [Google Scholar] [CrossRef] [PubMed]
- Baxamusa, S.H.; Gleason, K.K. Random copolymer films with molecular-scale compositional heterogeneities that interfere with protein adsorption. Adv. Funct. Mater. 2009, 19, 3489–3496. [Google Scholar] [CrossRef]
- Vladkova, T. Surface modification approach to control biofouling. Mar. Ind. Biofoul. 2009, 4, 135–163. [Google Scholar]
- Lu, Q.; Danner, E.; Waite, J.H.; Israelachvili, J.N.; Zeng, H.; Hwang, D.S. Adhesion of mussel foot proteins to different substrate surfaces. J. R. Soc. Interface 2013, 10, 1–11. [Google Scholar] [CrossRef]
- Holtenandersen, N.; Zhao, H.; Waite, J.H. Stiff coatings on compliant biofibers: The cuticle of mytilus californianus byssal threads. Biochemistry 2009, 48, 2752–2759. [Google Scholar] [CrossRef]
- Danielj, R.; Ali, M.; Herbertj, W. Diverse strategies of protein sclerotization in marine invertebrates: Structure-property relationships in natural biomaterials. Adv. Insect Physiol. 2010, 38, 75–133. [Google Scholar]
- Tian, Y.; Jiang, L. Wetting: Intrinsically robust hydrophobicity. Nat. Mater. 2013, 12, 291–292. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Liu, M.; Zhong, X.; Liu, G.; Wyman, I.; Wang, Z.; Wu, Y.; Yang, H.; Wang, J. Smooth water-based antismudge coatings for various substrates. ACS Sustain. Chem. Eng. 2017, 5, 2605–2613. [Google Scholar] [CrossRef]
- Zhang, W.; Yang, H.; Liu, F.; Chen, T.; Hu, G.; Guo, D.; Hou, Q.; Wu, X.; Su, Y.; Wang, J. Molecular interactions between DOPA and surfaces with different functional groups: A chemical force microscopy study. RSC Adv. 2017, 7, 32518–32527. [Google Scholar] [CrossRef]
- Rechendorff, K.; Hovgaard, M.B.; Foss, M.; Besenbacher, F. Influence of surface roughness on quartz crystal microbalance measurements in liquids. J. Appl. Phys. 2007, 101, 114502. [Google Scholar] [CrossRef]
- Chung, K.; Schumacher, J.; Sampson, E.; Burne, R.; Antonelli, P.; Brennan, A. Impact of engineered surface microtopography on biofilm formation of Staphylococcus aureus. Langmuir 1992, 8, 838–847. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, J.F.; Aldred, N.; Callow, M.E.; Finlay, J.A.; Callow, J.A.; Clare, A.S.; Brennan, A.B. Species-specific engineered antifouling topographies: Correlations between the settlement of algal zoospores and barnacle cyprids. Biofouling 2007, 23, 307–317. [Google Scholar] [CrossRef]
- Gopinathan, J.; Noh, I. Recent trends in bioinks for 3D printing. Biomaterials Research 2018, 22, 11. [Google Scholar] [CrossRef] [Green Version]
- Rebeiz, G.M.; Muldavin, J.B.; Schoenlinner, B.; Tan, G.L. RF MEMS: Theory, Design, and Technology; John Wiley & Sons: Hoboken, NJ, USA, 2004. [Google Scholar]
- Chang, J.; He, J.; Lei, Q.; Li, D. Electrohydrodynamic printing of microscale PEDOT: PSS-PEO features with tunable conductive/thermal properties. ACS Appl. Mater. Interfaces 2018, 10, 19116–19122. [Google Scholar] [CrossRef]
- Ramirez, J.C.C.; Tumolva, T.P. Analysis and optimization of water-based printing ink formulations for polyethylene films. Appl. Adhes. Sci. 2018, 6, 1–21. [Google Scholar] [CrossRef]
- Yu, D.; Yang, H.; Wang, H.; Cui, Y.; Yang, G.; Zhang, J.; Wang, J. Interactions between colloidal particles in the presence of an ultrahighly charged amphiphilic polyelectrolyte. Langmuir 2014, 30, 14512–14521. [Google Scholar] [CrossRef]
- Yang, H.; Duan, H.; Wu, X.; Wang, M.; Chen, T.; Liu, F.; Huang, S.; Zhang, W.; Chen, G.; Yu, D. Self-assembly behavior of ultrahighly charged amphiphilic polyelectrolyte on solid surfaces. Langmuir 2016, 32, 11485–11491. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Su, Y.; Liu, F.; Yang, H.; Wang, J. Study of interactions between 3,4-dihydroxyphenylalanine and surfaces with nano-, micro-and hierarchical structures using colloidal probe technology. Acta Physico-Chim. Sin. 2017, 33, 1644–1654. [Google Scholar]
- Hutter, J.L.; Bechhoefer, J. Calibration of atomic-force microscope tips. Rev. Sci. Instrum. 1993, 64, 1868–1873. [Google Scholar] [CrossRef]
- Lee, H.; Scherer, N.F.; Messersmith, P.B. Single-molecule mechanics of mussel adhesion. Proc. Natl. Acad. Sci. USA 2006, 103, 12999–13003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Qin, M.; Li, Y.; Cao, Y.; Wang, W. Single molecule evidence for the adaptive binding of DOPA to different wet surfaces. Langmuir 2014, 30, 4358–4366. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Wei, W.; Danner, E.; Israelachvili, J.N.; Waite, J.H. Effects of interfacial redox in mussel adhesive protein films on mica. Adv. Mater. 2011, 23, 2362–2366. [Google Scholar] [CrossRef] [PubMed]
- Sauerbrey, G. Use of vibrating quartz for thin film weighing and microweighing. Eur. Phys. J. A 1959, 155, 206–222. [Google Scholar]
- Huang, S.; Hou, Q.; Guo, D.; Yang, H.; Chen, T.; Liu, F.; Hu, G.; Zhang, M.; Zhang, J.; Wang, J. Adsorption mechanism of mussel-derived adhesive proteins onto various self-assembled monolayers. RSC Adv. 2017, 7, 39530–39538. [Google Scholar] [CrossRef] [Green Version]
Sample Availability: Samples of the functionalized surfaces are available from the authors. |






© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, H.; Zhang, W.; Chen, T.; Huang, S.; Quan, B.; Wang, M.; Li, J.; Gu, C.; Wang, J. Direct Experimental Evidence of Biomimetic Surfaces with Chemical Modifications Interfering with Adhesive Protein Adsorption. Molecules 2019, 24, 27. https://doi.org/10.3390/molecules24010027
Yang H, Zhang W, Chen T, Huang S, Quan B, Wang M, Li J, Gu C, Wang J. Direct Experimental Evidence of Biomimetic Surfaces with Chemical Modifications Interfering with Adhesive Protein Adsorption. Molecules. 2019; 24(1):27. https://doi.org/10.3390/molecules24010027
Chicago/Turabian StyleYang, Hui, Wei Zhang, Ting Chen, Shizhe Huang, Baogang Quan, Min Wang, Junjie Li, Changzhi Gu, and Jinben Wang. 2019. "Direct Experimental Evidence of Biomimetic Surfaces with Chemical Modifications Interfering with Adhesive Protein Adsorption" Molecules 24, no. 1: 27. https://doi.org/10.3390/molecules24010027