The Effect of Enzymolysis on Performance of Soy Protein-Based Adhesive
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
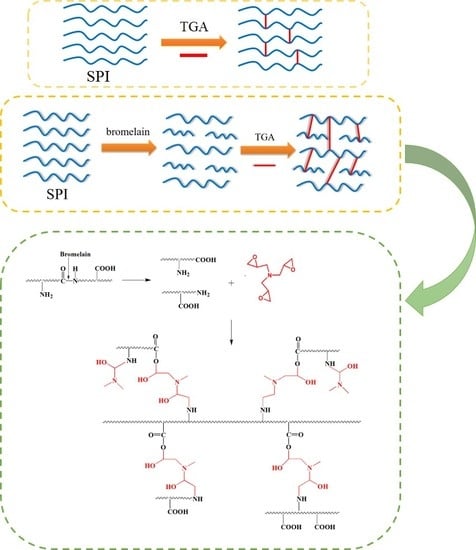
2.2. Preparation of Soy Protein Adhesive
2.3. Preparation and Evaluation of Plywood
2.4. Viscosity
2.5. Residual Rate Test
2.6. Wet Shear Strength Measurement
2.7. Fourier Transform Infrared (FTIR) Spectroscopy
2.8. Thermogravimetric (TG)
2.9. Scanning Electron Microscopy (SEM)
3. Results and Discussion
3.1. Viscosity
3.2. Residual Rate Test
3.3. Wet Shear Strength Measurement
3.4. Fourier Transform Infrared (FTIR) Spectroscopy Analysis
3.5. Thermogravimetric (TG) Analysis
3.6. Scanning Electron Microscopy (SEM) Analysis
4. Conclusions
- (1)
- Using 0.1 wt% bromelain effectively reduced the viscosity of the SPI adhesive by 67.9% and improved the SPI content of the adhesive from 12 wt% to 18 wt%, while maintaining a similar viscosity. After the enzymatic hydrolysis process, the residual rate of the SPI/bromelain adhesive markedly decreased by 9.6%, and the wet shear strength of the resultant plywood was reduced to 70.4%. These reductions were attributed to the breakdown of the soy protein molecules into polypeptide chains and the exposure of more hydrophilic groups.
- (2)
- With the addition of 9 wt% TGA, the residual rate of the SPI/bromelain/TGA adhesive improved by 13.7%, and the wet shear strength of the resultant plywood increased by 681.3% to 1.25 MPa, relative to that of the SPI/bromelain adhesive. This wet shear strength was 30.2% higher than that of the SPI/TGA adhesive. This improvement was attributed to the breakdown of soy protein molecules into polypeptide chains. This occurrence led to (1) the formation of more interlocks with the wood surface during the curing process of the adhesive and (2) the exposure of more hydrophilic groups and increase in the reactivity of protein with TGA, leading to a denser cross-linked network produced in the adhesive.
- (3)
- The formed cross-linked structure exhibited a higher thermal stability after enzymatic hydrolysis, indicating an improvement in the cross-link density of the adhesive. This structure also created a ductile fracture surface of the adhesive, indicating an improvement in the toughness of the adhesive.
Author Contributions
Funding
Conflicts of Interest
References
- Nordqvist, P.; Nordgren, N.; Khabbaz, F.; Malmström, E. Plant proteins as wood adhesives: Bonding performance at the macro- and nanoscale. Ind. Crop. Prod. 2013, 44, 246–252. [Google Scholar] [CrossRef]
- Vnučec, D.; Kutnar, A.; Goršek, A. Soy-based adhesives for wood-bonding—A review. J. Adhes. Sci. Technol. 2016, 31, 910–931. [Google Scholar] [CrossRef]
- Liu, H.; Li, C.; Sun, X.S. Improved water resistance in undecylenic acid (ua)-modified soy protein isolate (spi)-based adhesives. Ind. Crop. Prod. 2015, 74, 577–584. [Google Scholar] [CrossRef]
- Eslah, F.; Jonoobi, M.; Faezipour, M.; Ashori, A. Chemical modification of soybean flour-based adhesives using acetylated cellulose nanocrystals. Polym. Compos. 2017, 39, 3618–3625. [Google Scholar] [CrossRef]
- Li, J.; Luo, J.; Li, X.; Yi, Z.; Gao, Q.; Li, J. Soybean meal-based wood adhesive enhanced by ethylene glycol diglycidyl ether and diethylenetriamine. Ind. Crop. Prod. 2015, 74, 613–618. [Google Scholar] [CrossRef]
- Zhao, Y.; Xu, H.; Mu, B.; Xu, L.; Yang, Y. Biodegradable soy protein films with controllable water solubility and enhanced mechanical properties via graft polymerization. Polym. Degrad. Stab. 2016, 133, 75–84. [Google Scholar] [CrossRef] [Green Version]
- Kang, H.; Song, X.; Wang, Z.; Zhang, W.; Zhang, S.; Li, J. High-performance and fully renewable soy protein isolate-based film from microcrystalline cellulose via bio-inspired poly(dopamine) surface modification. ACS Sustain. Chem. Eng. 2016, 4, 4354–4360. [Google Scholar] [CrossRef]
- Jong, L. Reinforcement effect of soy protein nanoparticles in amine-modified natural rubber latex. Ind. Crop. Prod. 2017, 105, 53–62. [Google Scholar] [CrossRef]
- Eslah, F.; Jonoobi, M.; Faezipour, M.; Afsharpour, M.; Enayati, A.A. Preparation and development of a chemically modified bio-adhesive derived from soybean flour protein. Int. J. Adhes. Adhes. 2016, 71, 48–54. [Google Scholar] [CrossRef]
- Xu, F.; Dong, Y.; Zhang, W.; Zhang, S.; Li, L.; Li, J. Preparation of cross-linked soy protein isolate-based environmentally-friendly films enhanced by ptge and pam. Ind. Crop. Prod. 2015, 67, 373–380. [Google Scholar] [CrossRef]
- Luo, J.; Li, X.; Zhang, H.; Gao, Q.; Li, J. Properties of a soybean meal-based plywood adhesive modified by a commercial epoxy resin. Int. J. Adhes. Adhes. 2016, 71, 99–104. [Google Scholar] [CrossRef]
- Vnučec, D.; Mikuljan, M.; Kutnar, A.; Šernek, M.; Goršek, A. Influence of process parameters on the bonding performance of wood adhesive based on thermally modified soy proteins. Eur. J. Wood Wood Prod. 2016, 74, 553–561. [Google Scholar] [CrossRef]
- Nyanhongo, G.; Kudanga, T.; Prasetyo, E.N.; Guebitz, G.M. Mechanistic insights into laccase-mediated functionalisation of lignocellulose material. Biotechnol. Gen. Eng. 2010, 27, 305–330. [Google Scholar] [CrossRef] [Green Version]
- Slagman, S.; Zuilhof, H.; Franssen, M.C.R. Laccase-Mediated Grafting on Biopolymers and Synthetic Polymers: A Critical Review. ChemBioChem 2018, 19, 288–311. [Google Scholar] [CrossRef] [PubMed]
- Ribitsch, D.; Herrero Acero, E.; Greimel, K.; Dellacher, A.; Zitzenbacher, S.; Marold, A.; Rodriguez, R. A New Esterase from Thermobifida halotolerans Hydrolyses Polyethylene Terephthalate (PET) and Polylactic Acid (PLA). Polymers 2012, 4, 617–629. [Google Scholar] [CrossRef] [Green Version]
- Areskogh, D.; Henriksson, G. Immobilisation of laccase for polymerisation of commercial lignosulphonates. Process Biochem. 2011, 46, 1071–1075. [Google Scholar] [CrossRef]
- Lee, H.; Yildiz, G.; dos Santos, L.C.; Jiang, S.; Andrade, J.E.; Engeseth, N.J.; Feng, H. Soy protein nano-aggregates with improved functional properties prepared by sequential ph treatment and ultrasonication. Food Hydrocoll. 2016, 55, 200–209. [Google Scholar] [CrossRef]
- Luo, J.; Luo, J.; Li, X.; Li, K.; Gao, Q.; Li, J. Toughening improvement to a soybean meal-based bioadhesive using an interpenetrating acrylic emulsion network. J. Mater. Sci. 2016, 51, 9330–9341. [Google Scholar] [CrossRef]
- GB/T17657-2013. Standardization Administration of the People’s Republic of China. Available online: https://www.chinesestandard.net/PDF.aspx/GBT17657-2013 (accessed on 24 October 2018).
- Wang, X.; He, Z.; Zeng, M.; Qin, F.; Adhikari, B.; Chen, J. Effects of the size and content of protein aggregates on the rheological and structural properties of soy protein isolate emulsion gels induced by caso4. Food Chem. 2017, 221, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Cheng, E.Z.; Sun, X. Effects of wood-surface roughness, adhesive viscosity and processing pressure on adhesion strength of protein adhesive. J. Adhes. Sci. Technol. 2006, 20, 997–1017. [Google Scholar] [CrossRef]
- Gao, Q.; Qin, Z.Y.; Li, C.C.; Zhang, S.F.; Li, J.Z. Preparation of wood adhesives based on soybean meal modified with pegda as a crosslinker and viscosity reducer. Bioresources 2013, 8, 5380–5391. [Google Scholar] [CrossRef]
- Luo, J.; Luo, J.; Zhang, J.; Bai, Y.; Gao, Q.; Li, J.; Li, L. A new flexible soy-based adhesive enhanced with neopentyl glycol diglycidyl ether: Properties and application. Polymers 2016, 8, 346. [Google Scholar] [CrossRef]
- Luo, J.; Luo, J.L.; Yuan, C.; Zhang, W.; Li, J.Z.; Gao, Q.; Chen, H. An eco-friendly wood adhesive from soy protein and lignin: Performance properties. RSC Adv. 2015, 5, 100849–100855. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, Y.; Li, X.; Luo, J.; Gao, Q.; Li, J. “Green” bio-thermoset resins derived from soy protein isolate and condensed tannins. Ind. Crop. Prod. 2017, 108, 363–370. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, Y.; Li, X.N.; Luo, J.; Gao, Q.; Li, J.Z. A high-performance bio-adhesive derived from soy protein isolate and condensed tannins. RSC Adv. 2017, 7, 21226–21233. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Li, J.; Luo, J.; Li, K.; Gao, Q.; Li, J. A novel eco-friendly blood meal-based bio-adhesive: Preparation and performance. J. Polym. Environ. 2017, 26, 607–615. [Google Scholar] [CrossRef]
- Luo, J.; Li, L.; Luo, J.; Li, X.; Li, K.; Gao, Q. A high solid content bioadhesive derived from soybean meal and egg white: Preparation and properties. J. Polym. Environ. 2016, 25, 948–959. [Google Scholar] [CrossRef]
- Kumar, R.; Choudhary, V.; Mishra, S.; Varma, I.K. Enzymatically-modified soy protein part 2: Adhesion behaviour. J. Adhes. Sci. Technol. 2004, 18, 261–273. [Google Scholar] [CrossRef]
- Yuan, C.; Luo, J.; Luo, J.L.; Gao, Q.; Li, J.Z. A soybean meal-based wood adhesive improved by a diethylene glycol diglycidyl ether: Properties and performance. RSC Adv. 2016, 6, 74186–74194. [Google Scholar] [CrossRef]
- Luo, J.; Luo, J.L.; Bai, Y.Y.; Gao, Q.; Li, J.Z. A high performance soy protein-based bio-adhesive enhanced with a melamine/epichlorohydrin prepolymer and its application on plywood. RSC Adv. 2016, 6, 67669–67676. [Google Scholar] [CrossRef]
- Schmidt, V.; Giacomelli, C.; Soldi, V. Thermal stability of films formed by soy protein isolate–sodium dodecyl sulfate. Polym. Degrad. Stab. 2005, 87, 25–31. [Google Scholar] [CrossRef]
- Li, J.J.; Li, X.N.; Li, J.Z.; Gao, Q. Investigating the use of peanut meal: A potential new resource for wood adhesives. RSC Adv. 2015, 5, 80136–80141. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, W.; Lu, Y.; Gao, Z.; Gu, J. Nano-scale blocking mechanism of mmt and its effects on the properties of polyisocyanate-modified soybean protein adhesive. Ind. Crop. Prod. 2014, 57, 35–42. [Google Scholar] [CrossRef]
Sample Availability: Samples of the adhesive are available from the authors. |







| Sample | SPI (g) | Distilled Water (g) | Bromelain (g) | TGA (g) |
|---|---|---|---|---|
| 0 | 12 | 88 | 0 | 0 |
| 1 | 18 | 82 | 0.1 | 0 |
| 2 | 18 | 82 | 0.1 | 3 |
| 3 | 18 | 82 | 0.1 | 6 |
| 4 | 18 | 82 | 0.1 | 9 |
| 5 | 18 | 82 | 0.1 | 12 |
| 6 | 18 | 82 | 0 | 9 |
| Sample | 0 | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|---|
| Viscosity (cP) | 61,000 ± 2896 | 62,880 ± 3263 | 32,293 ± 1892 | 2439 ± 433 | 707 ± 82 | 285 ± 57 | 2200 ± 387 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Y.; Xu, Y.; Han, Y.; Chen, M.; Zhang, W.; Gao, Q.; Li, J. The Effect of Enzymolysis on Performance of Soy Protein-Based Adhesive. Molecules 2018, 23, 2752. https://doi.org/10.3390/molecules23112752
Xu Y, Xu Y, Han Y, Chen M, Zhang W, Gao Q, Li J. The Effect of Enzymolysis on Performance of Soy Protein-Based Adhesive. Molecules. 2018; 23(11):2752. https://doi.org/10.3390/molecules23112752
Chicago/Turabian StyleXu, Yantao, Yecheng Xu, Yufei Han, Mingsong Chen, Wei Zhang, Qiang Gao, and Jianzhang Li. 2018. "The Effect of Enzymolysis on Performance of Soy Protein-Based Adhesive" Molecules 23, no. 11: 2752. https://doi.org/10.3390/molecules23112752