Chemical Constituents from the Stems of Tinospora sinensis and Their Bioactivity
Abstract
:1. Introduction
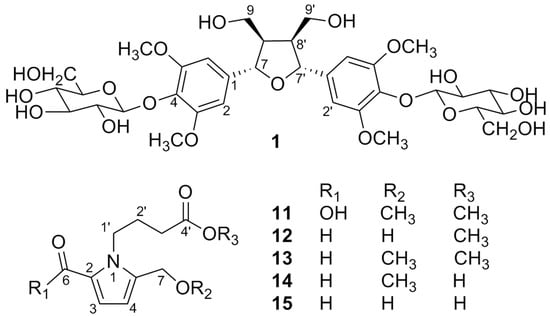
2. Results and Discussion
3. Materials and Methods
3.1. General Information
3.2. Materials
3.3. Extraction and Isolation
3.4. Anti-inflammatory Bioactivity Examination
3.4.1. Preparation of Human Neutrophils
3.4.2. Measurement of Superoxide Anion Generation and Elastase Release
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Witko-Sarsat, V.; Rieu, P.; Descamps-Latscha, B.; Lesavre, P.; Halbwachs-Mecarelli, L. Neutrophils: Molecules, functions and pathophysiological aspects. Lab. InvestIG. 2000, 80, 617–653. [Google Scholar] [CrossRef] [PubMed]
- Okajima, K.; Harada, N.; Uchiba, M. Ranitidine Reduces Ischemia/Reperfusion-Induced Liver Injury in Rats by Inhibiting Neutrophil Activation. J. Pharmacol. Exp. Ther. 2002, 301, 1157–1165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ennis, M. Neutrophils in asthma pathophysiology. Curr. Allergy Asthma Rep. 2003, 3, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Hwang, T.L.; Li, G.L.; Lan, Y.H.; Chia, Y.C.; Hsieh, P.W.; Wu, Y.H.; Wu, Y.C. Potent inhibitors of superoxide anion production in activated human neutrophils by isopedicin, a bioactive component of the Chinese medicinal herb Fissistigma oldhamii. Free Radic. Biol. Med. 2009, 46, 520–528. [Google Scholar] [CrossRef] [PubMed]
- Malech, H.L.; Gallin, J.I. Current concepts: Immunology: Neutrophils in human diseases. N. Engl. J. Med. 1987, 317, 687–694. [Google Scholar] [CrossRef] [PubMed]
- Van Eeden, S.F.; Klut, M.E.; Walker, B.A.M.; Hogg, J.C. The use of flow cytometry to measure neutrophil function. J. Immunol. Methods 1999, 232, 23–43. [Google Scholar] [CrossRef]
- Editorial Committee of the Flora of Taiwan. Flora of Taiwan, 2nd ed.; Department of Botany, National Taiwan University: Taipei, Taiwan, 1996; Volume 2, p. 605. [Google Scholar]
- Krishna, K.L.; Jigar, B.; Jagruti, P. Guduchi (Tinospora cordifolia): Biological and Medicinal properties: A review. Int. J. Altern. Med. 2009, 6, 1–12. [Google Scholar]
- Mishra, A.; Kumar, S.; Bhargava, A.; Sharma, B.; Pandey, A.K. Studies on in vitro antioxidant and antistaphylococcal activities of some important medicinal plants. Cell Mol. Biol. 2011, 57, 16–25. [Google Scholar] [PubMed]
- Upadhyay, A.K.; Kumar, K.; Kumar, A.; Mishra, H.S. Tinospora cordifolia (Willd.) Hook. f. and Thoms. (Guduchi)–validation of the Ayurvedic pharmacology through experimental and clinical studies. Int. J. Ayurveda Res. 2010, 1, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Velazquez, E.A.; Kimura, D.; Torbati, D.; Ramachandran, C.; Totapally, B.R. Immunological response to (1,4)-α-D-glucan in the lung and spleen of endotoxin-stimulated juvenile rats. Basic Clin. Pharmacol. Toxicol. 2009, 105, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Lam, S.H.; Ruan, C.T.; Hsieh, P.H.; Su, M.J.; Lee, S.S. Hypoglycemic Diterpenoids from Tinospora crispa. J. Nat. Prod. 2012, 75, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Ruan, C.T.; Lam, S.H.; Lee, S.S.; Su, M.J. Hypoglycemic action of borapetoside A from the plant Tinospora crispa in mice. Phytomedicine 2013, 20, 667–675. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.H.; Akao, T.; Hamasaki, K.; Deyama, T.; Hattori, M. Biotransformation of pinoresinol diglucoside to mammalian lignans by human intestinal microflora, and isolation of Enterococcus faecalis strain PDG-1 responsible for the transformation of (+)-pinoresinol to (+)-lariciresinol. Chem. Pharm. Bull. 2003, 51, 508–515. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Wu, T.Y.; Chang, F.R.; Wu, Y.C. Lignans and kauranes from the stems of Annona cherimola. J. Chin. Chem. Soc. 1998, 45, 629–634. [Google Scholar] [CrossRef]
- Deyama, T. The constituents of Eucommia ulmoides Oliv. I. Isolation of (+)-medioresinol di-O-β-D- glucopyranoside. Chem. Pharm. Bull. 1983, 31, 2993–2997. [Google Scholar] [CrossRef]
- Kitagawa, S.; Nishibe, S.; Benecke, R.; Thieme, H. Phenolic compounds from Forsythia leaves. II. Chem. Pharm. Bull. 1988, 36, 3667–3670. [Google Scholar] [CrossRef]
- Kinjo, J.; Higuchi, H.; Fukui, K.; Nohara, T. Lignoids from Albizziae cortex. II. A biodegradation pathway of syringaresinol. Chem. Pharm. Bull. 1991, 39, 2952–2955. [Google Scholar] [CrossRef]
- Jong, T.T.; Jean, M.Y. Constituents of Houttuynia cordata and the crystal structure of vomifoliol. J. Chin. Chem. Soc. 1993, 40, 399–402. [Google Scholar] [CrossRef]
- Haslam, E. The stereochemistry of sesamolin. J. Chem. Soc. C 1970, 17, 2332–2334. [Google Scholar] [CrossRef]
- Sudhakar, G.; Kadam, V.D.; Bayya, S.; Pranitha, G.; Jagadeesh, B. Total synthesis and stereochemical revision of acortatarins A and B. Org. Lett. 2011, 13, 5452–5455. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.B.; Chang, B.Y.; Hwang, B.Y.; Kim, S.Y.; Lee, M.K. Pyrrole alkaloids from the fruits of Morus alba. Bioorg. Med. Chem. Lett. 2014, 24, 5656–5659. [Google Scholar] [CrossRef] [PubMed]
- Chin, Y.W.; Lim, S.W.; Kim, S.H.; Shin, D.Y.; Suh, Y.G.; Kim, Y.B.; Kim, Y.C.; Kim, J. Hepatoprotective Pyrrole Derivatives of Lycium chinense Fruits. Bioorg. Med. Chem. Lett. 2003, 13, 79–81. [Google Scholar] [CrossRef]
- Zhou, J.T.; Li, C.Y.; Wang, C.H.; Wang, Y.F.; Wang, X.D.; Wang, H.T.; Zhu, Y.; Jiang, M.M.; Gao, X.M. Phenolic Compounds from the Roots of Rhodiola crenulata and Their Antioxidant and Inducing IFN-γ Production Activities. Molecules 2015, 20, 13725–13739. [Google Scholar] [CrossRef] [PubMed]
- Gopalakrishnan, S.; Subbarao, G.V.; Nakahara, K.; Yoshihashi, T.; Ito, O.; Maeda, I.; Ono, H.; Yoshida, M. Nitrification inhibitors from the root tissues of Brachiaria humidicola, a tropical grass. J. Agric. Food Chem. 2007, 55, 1385–1388. [Google Scholar] [CrossRef] [PubMed]
- Okuyama, E.; Suzumura, K.; Yamazaki, M. Pharmacologically active components of Todopon Puok (Fagraea racemosa), a medicinal plant from Borneo. Chem. Pharm. Bull. 1995, 43, 2200–2204. [Google Scholar] [CrossRef] [PubMed]
- Olesch, B.; Böhm, H. Abbau des 2-benzyl-isochinolin-alkaloids sendaverin. Arch. Pharm. 1972, 305, 222–229. [Google Scholar] [CrossRef]
- Lee, S.Y.; Choi, S.U.; Lee, J.H.; Lee, D.U.; Lee, K.R. A new phenylpropane glycoside from the rhizome of Sparganium stoloniferum. Arch. Pharm. Res. 2010, 33, 515–521. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.M.; Liu, Y.L.; Li, X.R.; Feng, Y.L.; Yang, S.L. Two new phenylglycol derivatives isolated from Syringa reticulata var. mandshurica and their antifungal activities. Chem. Pharm. Bull. 2009, 57, 863–866. [Google Scholar] [PubMed]
- Zhong, X.N.; Otsuka, H.; Ide, T.; Hirata, E.; Takeda, Y. Hydroquinone diglycoside acyl esters from the leaves of Myrsine seguinii. Phytochemistry 1999, 52, 923–927. [Google Scholar] [CrossRef]
- Miyase, T.; Ueno, A.; Takizawa, N.; Kobayashi, H.; Oguchi, H. Ionone and lignan glycosides from Epimedium diphyllum. Phytochemistry 1989, 28, 3483–3485. [Google Scholar] [CrossRef]
- Kuwajima, H.; Takai, Y.; Takaishi, K.; Inoue, K. Synthesis of 13C-labeled possible intermediates in the biosynthesis of phenylethanoid derivatives, cornoside and rengyosides. Chem. Pharm. Bull. 1998, 46, 581–586. [Google Scholar] [CrossRef]
- Greca, M.D.; Ferrara, M.; Fiorentino, A.; Monaco, P.; Previtera, L. Antialgal compounds from Zantedeschia aethiopica. Phytochemistry 1998, 49, 1299–1304. [Google Scholar] [CrossRef]
- Maurya, R.; Wazir, V.; Tyagi, A.; Kapil, R.S. Cordifoliosides A and B, two new phenylpropene disaccharides from Tinospora cordifolia possessing immunostimulant activity. Nat. Prod. Lett. 1996, 8, 7–10. [Google Scholar] [CrossRef]
- Chen, C.Y.; Chang, F.R.; Teng, C.M.; Wu, Y.C. Cheritamine, a new N-fatty acyl tryptamine and other constituents from the stems of Annona cherimola. J. Chin. Chem. Soc. 1999, 46, 77–86. [Google Scholar] [CrossRef]
- Li, A.; Mishra, Y.; Malik, M.; Wang, Q.; Li, S.; Taylor, M.; Reichert, D.E.; Luedtke, R.R.; Mach, R.H. Evaluation of N-phenyl homopiperazine analogs as potential dopamine D3 receptor selective ligands. Bioorg. Med. Chem. 2013, 21, 2988–2998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.L.; Li, Y.J.; Wang, A.M.; He, X.; Liao, S.G.; Lan, Y.Y. Two new phenolic glycosides from Inula cappa. J. Asian Nat. Prod. Res. 2010, 12, 765–769. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.K.; Lee, P.H.; Kuo, Y.H. The chemical constituents from the aril of Cassia fistula L. J. Chin. Chem. Soc. 2001, 48, 1053–1058. [Google Scholar] [CrossRef]
- Chang, Y.C.; Chang, F.R.; Wu, Y.C. The constituents of Lindera glauca. J. Chin. Chem. Soc. 2000, 47, 373–380. [Google Scholar] [CrossRef]
- Wada, T. Structure of digiprolactone. Chem. Pharm. Bull. 1965, 13, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Saxena, S.; Tripathi, J.; Chatterjee, S.; Gautam, S. Natural predominance of abscisic acid in Pongammia pinnata ("Karanj") honey contributed to its strong antimutagenicity. J. Agric. Food Chem. 2017, 65, 4624–4633. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, A.D.; Acosta, A.L. New cembranoid diterpenes and a geranylgeraniol derivative from the common Caribbean sea whip Eunicea succinea. J. Nat. Prod. 1997, 60, 1134–1138. [Google Scholar] [CrossRef] [PubMed]
- Atta-ur-Rahman; Ahmad, S.; Rycroft, D.S.; Prknyi, L.; Choudhary, M.I.; Clardy, J. Malabarolide, a novel furanoid bisnorditerpenoid from Tinospora malabarica. Tetrahedron Lett. 1988, 29, 4241–4244. [Google Scholar] [CrossRef]
- Fotie, J.; Bohle, D.S.; Leimanis, M.L.; Georges, E.; Rukunga, G.; Nkengfack, A.E. Lupeol long-chain fatty acid esters with antimalarial activity from Holarrhena floribunda. J. Nat. Prod. 2006, 69, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.C.; Desjardins, A.E.; Wu, C.D.; Kinghorn, A.D. Activity of triterpenoid glycosides from the root bark of Mussaenda macrophylla against two oral pathogens. J. Nat. Prod. 1999, 62, 1379–1384. [Google Scholar] [CrossRef] [PubMed]
- Kocór, M.; St. Pyrek, J. Cyclotrichosantol, a new C31 31-nortriterpene. J. Org. Chem. 1973, 38, 3688–3690. [Google Scholar]
- Baldé, A.M.; Apers, S.; Claeys, M.; Pieters, L.; Vlietinck, A.J. Cycloabyssinone, a new cycloterpene from Harrisonia abyssinica. Fitoterapia 2001, 72, 438–440. [Google Scholar] [CrossRef]
- Kikuchi, T.; Toyoda, T.; Arimoto, M.; Takayama, M.; Yamano, M. Studies on the neutral constituents of Pachysandra terminalis Sieb. et Zucc. I. Isolation and characterization of sterols and triterpenes. Yakugaku Zasshi 1969, 89, 1358–1366. [Google Scholar] [CrossRef] [PubMed]
- Öksüz, S.; Shieh, H.L.; Pezzuto, J.M.; Özhatay, N.; Cordell, G.A. Biologically active compounds from the Euphorbiaceae; part 1. Triterpenoids of Euphorbia nicaeensis subsp. glareosa. Planta Med. 1993, 59, 472–473. [Google Scholar]
- Kuo, Y.H.; Li, Y.C. Constituents of the bark of Ficus microcarpa L. f. J. Chin. Chem. Soc. 1997, 44, 321–325. [Google Scholar] [CrossRef]
- Kimura, Y.; Yasukawa, K.; Takido, M.; Akihisa, T.; Tamura, T. Inhibitory effect of some oxygenated stigmastane-type sterols on 12-O-tetradecanoylphorbol-13-acetate-induced inflammation in mice. Biol. Pharm. Bull. 1995, 18, 1617–1619. [Google Scholar] [CrossRef] [PubMed]
- Katsui, N.; Matsue, H.; Hirata, T.; Masamune, T. Phytosterols and triterpenes in roots of the “kidney bean” (Phaseolus vulgaris L.). Bull. Chem. Soc. Jpn. 1972, 45, 223–226. [Google Scholar] [CrossRef]
- Zhang, X.; Geoffroy, P.; Miesch, M.; Julien-David, D.; Raul, F.; Aoudé-Werner, D.; Marchioni, E. Gram-scale chromatographic purification of beta-sitosterol. Synthesis and characterization of beta-sitosterol oxides. Steroids 2005, 70, 886–895. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.J.; Tsai, I.L.; Ishikawa, T.; Wang, C.J.; Chen, I.S. Alkaloids from trunk bark of Hernandia nymphaeifolia. Phytochemistry 1996, 42, 1479–1484. [Google Scholar] [CrossRef]
- Tseng, C.F.; Iwakama, S.; Mikajiri, A.; Shibuya, M.; Hanaoka, F.; Ebizuka, Y.; Padmawinata, K.; Sankawa, U. Inhibition of in vitro prostaglandin and leukotriene biosyntheses by cinnamoyl-β-phenethylamine and N-acyldopamine derivatives. Chem. Pharm. Bull. 1992, 40, 396–400. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, T.J.; Chang, F.R.; Wu, Y.C. The constituents of Cananga odorata. J. Chin. Chem. Soc. 1999, 46, 607–611. [Google Scholar] [CrossRef]
- Yoshihara, T.; Yamaguchi, K.; Takamatsu, S.; Sakamura, S. A new lignan amide, grossamide, from bell pepper (Capsicum annuum var. grossum). Agric. Biol. Chem. 1981, 45, 2593–2598. [Google Scholar]
- Bayoumi, S.A.L.; Rowan, M.G.; Beeching, J.R.; Blagbrough, I.S. Constituents and secondary metabolite natural products in fresh and deteriorated cassava roots. Phytochemistry 2010, 71, 598–604. [Google Scholar] [CrossRef] [PubMed]
- Harris, T.M.; Hay, J.V. Biogenetically modeled syntheses of heptaacetate metabolites. Alternariol and lichexanthone. J. Am. Chem. Soc. 1977, 99, 1631–1636. [Google Scholar] [CrossRef]
- Nishina, A.; Hasegawa, K.; Uchibori, T.; Seino, H.; Osawa, T. 2,6-Dimethoxy-p-benzoquinone as an antibacterial substance in the bark of Phyllostachys heterocycla var. pubescens, a species of thick-stemmed bamboo. J. Agric. Food Chem. 1991, 39, 266–269. [Google Scholar] [CrossRef]
- Kanchanapoom, T.; Kamel, M.S.; Kasai, R.; Yamasaki, K.; Picheansoonthon, C.; Hiraga, Y. Lignan glucosides from Acanthus ilicifolius. Phytochemistry 2001, 56, 369–372. [Google Scholar] [CrossRef]
- Kim, S.B.; Chang, B.Y.; Jo, Y.H.; Lee, S.H.; Han, S.B.; Hwang, B.Y.; Kim, S.Y.; Lee, M.K. Macrophage activating activity of pyrrole alkaloids from Morus alba fruits. J. Ethnopharmacol. 2013, 145, 393–396. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.C.; Chung, P.J.; Ho, C.M.; Kuo, C.Y.; Hung, M.F.; Huang, Y.T.; Chang, W.Y.; Chang, Y.W.; Chan, K.H.; Hwang, T.L. Propofol Inhibits Superoxide Production, Elastase Release, and Chemotaxis in Formyl Peptide–Activated Human Neutrophils by Blocking Formyl Peptide Receptor 1. J. Immunol. 2013, 190, 6511–6519. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.P.; Hsieh, P.W.; Chang, Y.J.; Chung, P.J.; Kuo, L.M.; Hwang, T.L. 2-(2-Fluoro-benzamido)benzoate ethyl ester (EFB-1) inhibits superoxide production by human neutrophils and attenuates hemorrhagic shock-induced organ dysfunction in rats. Free Radic. Biol. Med. 2011, 50, 1737–1748. [Google Scholar] [CrossRef] [PubMed]
- Moody, J.O.; Robert, V.A.; Connolly, J.D.; Houghton, P.J. Anti-inflammatory activities of the methanol extracts and an isolated furanoditerpene constituent of Sphenocentrum jollyanum Pierre (Menispermaceae). J. Ethnopharmacol. 2006, 104, 87–91. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of all the isolated compounds are available from the authors. |




| Position | 1 a | 17 b | 16 b | |||
|---|---|---|---|---|---|---|
| δH | δC | HMBC (H→C) | δH | δH | δC | |
| 1 | 133.7 s | 127.0 s | ||||
| 2 | 6.66 s | 104.2 d | 85.0, 104.2, 137.1, 152.6 | 7.76 d (1.9) | 7.04 d (1.8) | 109.3 d |
| 3 | 152.6 s | 146.6 s | ||||
| 4 | 137.1 s | 147.9 s | ||||
| 5 | 152.6 s | 6.88 d (8.3) | 6.92 d (8.2) | 112.7 d | ||
| 6 | 6.66 s | 104.2 d | 85.0, 104.2, 137.1, 152.6 | 7.10 dd (8.3, 1.9) | 7.07 dd (8.2, 1.8) | 123.1 d |
| 7 | 4.66 br d (3.8) | 85.0 d | 53.6, 71.3, 104.2, 137.1 | 6.80 d (12.9) | 7.59 d (16.0) | 144.8 d |
| 8 | 3.09 m | 53.6 d | 5.81 d (12.9) | 6.28 d (16.0) | 115.5 d | |
| 9 | 3.84 dd (9.0, 3.2) | 71.3 t | 53.6, 85.0 | 167.5 s | ||
| 4.18 dd (9.0, 6.7) | 53.6, 85.0, 104.2 | |||||
| 1′ | 133.7 s | 173.9 s | ||||
| 2′ | 6.66 s | 104.2 d | 85.0, 104.2, 137.1, 152.6 | 2.31 t (7.6) | 2.34 t (7.4) | 33.8 t |
| 3′ | 152.6 s | 1.66 m | 1.67 m | 24.5 t | ||
| 4′ | 137.1 s | 1.37 m | 1.47 m | 25.3 t | ||
| 5′ | 152.6 s | 1.66 m | 1.67 m | 28.1 t | ||
| 6′ | 6.66 s | 104.2 d | 85.0, 104.2, 137.1, 152.6 | 4.12 t (6.6) | 4.19 t (6.6) | 64.0 t |
| 7′ | 4.66 br d (3.8) | 85.0 d | 53.6, 71.3, 104.2, 137.1 | |||
| 8′ | 3.09 m | 53.6 d | ||||
| 9′ | 3.84 dd (9.0, 3.2) | 71.3 t | 53.6, 85.0 | |||
| 4.18 dd (9.0, 6.7) | 53.6, 85.0, 104.2 | |||||
| Bz-OMe | 3.76 s | 56.4 q | 3.93 s | 3.95 s | 55.9 q | |
| OMe | 3.67 s | 3.67 s | 51.5 q | |||
| Glc H1, 1′ | 4.90 d (5.2) | 102.6 d | 76.5, 74.1 | |||
| Glc H2, 2′ | 3.17 m | 76.5 d | 74.1 | |||
| Glc H3, 3′ | 3.17 m | 74.1 d | 76.5 | |||
| Glc H4, 4′ | 3.11 m | 69.9 d | 76.5 | |||
| Glc H5, 5′ | 3.02 m | 77.2 d | 69.9 | |||
| Glc H6, 6′ | 3.40 m | 60.9 t | 77.2 | |||
| 3.59 m | ||||||
| Position | 11 a | 12 b | 13 a | 14 c | 15 c | ||
|---|---|---|---|---|---|---|---|
| δH | δC | δH | δC | δH | δH | δH | |
| 2 | 121.6 s | 133.5 s | |||||
| 3 | 7.01 d (3.9) | 119.0 d | 6.98 d (4.0) | 126.5 d | 6.87 d (4.0) | 6.96 d (4.0) | 6.97 d (4.0) |
| 4 | 6.16 d (3.9) | 110.8 d | 6.26 d (4.0) | 111.5 d | 6.23 d (4.0) | 6.27 d (4.0) | 6.25 d (4.0) |
| 5 | 136.9 s | 144.6 s | |||||
| 6 | 162.2 s | 9.42 s | 180.9 d | 9.50 s | 9.45 s | 9.40 s | |
| 7 | 4.43 s | 65.8 t | 4.63 s | 56.4 t | 4.45 s | 4.52 s | 4.65 s |
| 1′ | 4.37 br t (7.6) | 44.7 t | 4.38 dd (7.4, 6.0) | 45.7 t | 4.36 br t (7.6) | 4.35 br t (7.6) | 4.37 dd (7.5, 6.0) |
| 2′ | 2.04 m | 26.5 t | 2.01 m | 27.5 t | 2.01 m | 1.96 m | 1.98 m |
| 3′ | 2.36 t (7.3) | 31.0 t | 2.35 t (7.3) | 31.6 t | 2.36 t (7.2) | 2.23 t (7.5) | 2.27 t (7.5) |
| 4′ | 173.4 s | 175.1 s | |||||
| OCH3 | 3.67 s | 57.7 q | 3.66 s | 52.2 q | 3.68 s | ||
| CH2OCH3 | 3.34 s | 51.6 q | 3.36 s | 3.36 s | |||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lam, S.-H.; Chen, P.-H.; Hung, H.-Y.; Hwang, T.-L.; Chiang, C.-C.; Thang, T.D.; Kuo, P.-C.; Wu, T.-S. Chemical Constituents from the Stems of Tinospora sinensis and Their Bioactivity. Molecules 2018, 23, 2541. https://doi.org/10.3390/molecules23102541
Lam S-H, Chen P-H, Hung H-Y, Hwang T-L, Chiang C-C, Thang TD, Kuo P-C, Wu T-S. Chemical Constituents from the Stems of Tinospora sinensis and Their Bioactivity. Molecules. 2018; 23(10):2541. https://doi.org/10.3390/molecules23102541
Chicago/Turabian StyleLam, Sio-Hong, Po-Hsun Chen, Hsin-Yi Hung, Tsong-Long Hwang, Chih-Chao Chiang, Tran Dinh Thang, Ping-Chung Kuo, and Tian-Shung Wu. 2018. "Chemical Constituents from the Stems of Tinospora sinensis and Their Bioactivity" Molecules 23, no. 10: 2541. https://doi.org/10.3390/molecules23102541