Aqueous Extract of Perilla frutescens var. acuta Relaxes the Ciliary Smooth Muscle by Increasing NO/cGMP Content In Vitro and In Vivo
Abstract
:1. Introduction
2. Results and Discussion
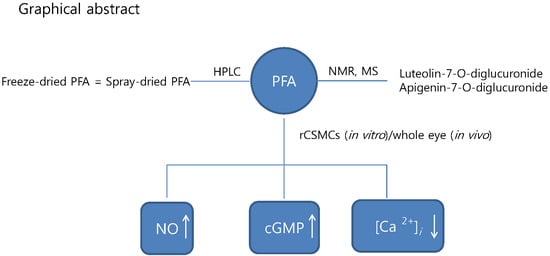
2.1. Preparation and Characterization of PFA
2.2. HPLC Analysis of Freeze-Dried and Spray-Dried Samples of PFA
2.3. Effect of the Freeze-Dried PFA on NO Production in rCSMCs
2.4. Effect of the Freeze-Dried PFA on the Production of cAMP and cGMP in rCSMCs
2.5. Effect of the Freeze-Dried PFA Extract on [Ca2+]i in rCSMCs
2.6. Effect of the Freeze-Dried PFA Extract on the Production of cGMP In Vivo
3. Materials and Methods
3.1. Materials
3.2. Extraction and Drying Process
3.3. Indentification of Compounds
3.4. HPLC Analysis
3.5. Preparation of Tissue and Cell Culture
3.6. Cell Viability
3.7. Determination of NO Production
3.8. Measurement of cGMP and cAMP Production
3.9. Measurement of [Ca2+]i
3.10. Measurement of cGMP Production In Vivo
3.11. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lee, C.W.; Choi, H.M.; Kim, S.Y.; Lee, J.R.; JO, C. Influence of Perilla frutescens var. acuta water extract on the shelf life and physicochemical qualities of cooked beef patties. Korean J. Food Sci. Anim. Resour. 2015, 35, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.Y.; Choi, H.J.; Chung, T.W.; Choi, J.Y.; Kim, H.S.; Jung, Y.S.; Lee, S.O.; Ha, K.T. Water-extracted Perilla frutescens increases endometrial receptivity though leukemia inhibitory factor-dependent expression of integrins. J. Pharmacol. Sci. 2016, 131, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Asada, T.; Sato, A.; Koi, Y.; Nishiwaki, H.; Tamura, H. Rosmarinic acid extract for antioxidant, antiallergic, and glucosidase inhibitory activities, isolated by supramolecular technique and solvent extraction from Perilla leaves. J. Agric. Food Chem. 2014, 62, 885–892. [Google Scholar] [CrossRef] [PubMed]
- Jun, H.I.; Kim, B.T.; Song, G.S.; Kim, Y.S. Structural characterization of phenolic antioxidants from purple perilla (Perilla frutescens var. acuta) leaves. Food Chem. 2014, 148, 367–372. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Loazno, Y.F.; Gaydou, E.M.; Li, B. Antioxidant activities of polyphenols extracted from Perilla frutescens varieties. Molecules 2009, 14, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Banno, N.; Akihisa, T.; Tokuda, H.; Yasukawa, K.; Hiqashihara, H.; Watanabe, K.; Kimura, Y.; Haseqawa, J.; Nishino, H. Triterpene acids from the leaves of Perilla frutescens and their anti-inflammatory and anti-tumor-promoting effects. Biosci. Biotechnol. Biochem. 2004, 68, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Oh, H.A.; Park, C.S.; Ahn, H.J.; Park, Y.S.; Kim, H.M. Effect of Perilla frutescens var. acuta Kudo and rosmarinic acid on allergic inflammatory reactions. Exp. Biol. Med. 2011, 236, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Kang, R.; Helms, R.; Stout, M.J.; Jaber, H.; Chen, Z.; Nakatsu, T. Antimicrobial activity of the volatile constituents of Perilla frutescens and its synergistic effects with polygodial. J. Agric. Food Chem. 1992, 40, 2328–2330. [Google Scholar] [CrossRef]
- Choi, S.H.; Hur, J.M.; Yang, E.J.; Jun, M.; Park, H.J.; Lee, K.B.; Moon, E.; Song, K.S. Beta-secretase (BACE1) inhibitors from Perilla frutescens var. acuta. Arch. Pharm. Res. 2008, 31, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Takeda, H.; Tsuji, M.; Matsumiya, T.; Kubo, M. Identification of rosmarinic acid as a novel antidepressive substance in the leaves of Perilla frutescens Britton var. acuta Kudo (Perillae Herba). Nihon Shinke Seishin Yakuriqaku Zasshi 2002, 22, 15–22. [Google Scholar]
- Meng, L.; Lozano, Y.; Bombarda, I.; Gaydou, E.M.; Li, B. Polyphenol extraction from eight Perilla frutescens cultivars. C. R. Chim. 2009, 12, 602–611. [Google Scholar] [CrossRef]
- Yoshihara, A.; Yamanaka, K.; Kawakami, M. Effects of polyphenol on visual fatigue caused by VDT work. Int. J. Occup. Saf. Ergon. 2009, 15, 339–343. [Google Scholar] [CrossRef] [PubMed]
- Parihar, J.K.; Jain, V.K.; Chaturvedi, P.; Kaushik, J.; Jain, G.; Parihar, K.S. Computer and visual display terminals (VDT) vision syndrome (CVDTS). Med. J. Armed Forces India 2016, 72, 270–276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ozaway, Y.; Kawashima, M.; Inoue, S.; Inaqaki, E.; Suzuki, A.; Ooe, E.; Kobayashi, S.; Tsubota, K. Bilberry extract supplementation for preventing eye fatigue in video display terminal workers. J. Nutr. Health Aging 2015, 19, 548–554. [Google Scholar] [CrossRef] [PubMed]
- Bogdănici, C.M.; Săndulache, D.E.; Nechita, C.A. Eyesight quality and computer vision syndrome. Rom. J. Ophthalmol. 2017, 61, 112–116. [Google Scholar] [CrossRef] [PubMed]
- Iwao, K.; Tsutomu, A. A novel in in vitro model for screening and evaluation of anti-asthenopia drug. J. Pharmacol. Sci. 2003, 93, 222–224. [Google Scholar]
- Sagawa, K.; Hashimoto, K.; Kawada, S.; Yagi, S.; Yamaguchi, H. Protective effect of bilberry-derived anthocyanins-containing diet on VDT work-induced subjective asthenopic symptoms and the accommodative eye function impairment in healthy adults. Pharmacol. Ther. 2013, 41, 155–165. [Google Scholar]
- Kono, K.; Shimizu, Y.; Takahashi, S.; Matsuoka, S.; Yui, K. Effect of multiple dietary supplement containing lutein, astaxanthin, cyaniding-3-glucoside, and DHA on accommodative ability. Curr. Med. Chem. 2014, 14, 114–125. [Google Scholar]
- Sakimoto, T.; Kojima, Y.; Kashiwabara, Y.; Chikui, C. Clinical study with black soybean extract on ocular function. Jpn. Rev. Clin. Ophthalmol. 2005, 98, 982–986. [Google Scholar]
- Nagaki, Y.; Minhara, Y.; Tsukahara, H.; Ohno, S. Supplementation effects of astaxanthin on accommodation and asthenopia. J. Clin. Ther. Med. 2006, 14, 114–125. [Google Scholar]
- Beauregard, C.; Liu, Q.; Chiou, G. Effects of nitiric oxide donors and nitric oxide synthase substrates on ciliary muscle contracted by carbachol and endothelin for possible use in myopia prevention. J. Ocul. Pharmacol. 2001, 17, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Chiou, G. Review: Effects of nitric oxide on eye diseases and their treatment. J. Ocul. Pharmacol. 2001, 17, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Kamikawatoko, S.; Tokoro, T.; Ishida, A.; Masuda, H.; Jun sato, H.H.; Azuma, H. Nitric oxide relaxes bovine ciliary muscle contracted by carbachol through elevation of cyclic GMP. Exp. Eye Res. 1998, 66, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Carreiro, S.; Anderson, S.; Gukasyan, H.J.; Krauss, A.; Prasanna, G. Correlation of in vitro and in vivo kinetics of nitric oxide donors in ocular tissues. J. Ocul. Pharmacol. Ther. 2009, 25, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Gabelt, B.T.; Kaufman, P.L.; Rasmussen, C.A. Effect of nitric oxide compounds on monkey ciliary muscle in vitro. Exp. Eye Res. 2011, 93, 321–327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimura, M.; Yasuda, K.; Nakzawa, T.; Kashiwagi, K. The effect of unoprostone isopropyl on Ca2+ release-activated Ca2+ currents in cultured monkey trabecular meshwork cells and ciliary muscle cells. J. Ocul. Pharmacol. Ther. 2006, 22, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, S.; Yorio, T.; DeSantis, L.; Pang, I.H. Muscarinic effects on cellular functions in cultured human ciliary muscle cells. Investig. Ophthalmol. Vis. Sci. 1994, 35, 3732–3738. [Google Scholar]
- Jeong, K.I.; Ryu, G.C. An effect of extract of Perilla frutescens britton var. acuta kudo on changes in refractive error. Korea J. Vis. Sci. 2016, 18, 167–174. [Google Scholar] [CrossRef]
- Jeong, K.I.; Kim, J.; Choi, C.Y.; Ryu, G.C. Effects of Perilla frutescens var. acuta aqueous extract on the visual function. Korea J. Vis. Sci. 2017, 19, 283–291. [Google Scholar] [CrossRef]
- Correia, R.; Grace, M.H.; Esposito, D.; Lila, M.A. Wild blueberry polyphenol-protein food ingredients produced by three drying methods: Comparative physico-chemical properties, phytochemical content, and stability during storage. Food Chem. 2017, 235, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xue, X.; Xiao, Y.; Zhang, F.; Xu, Q.; Liang, X. Purification and preparation of compounds from an extract of Scutellaria barbata D.Don using preparative parallel high performance liquid chromatography. J. Sep. Sci. 2008, 31, 1669–1676. [Google Scholar]
- Yoshida, K.; Kameda, K.; Kondo, T. Diglucuronoflavones from purple leaves of Perilla ocimoides. Phytochemistry 1993, 33, 917–919. [Google Scholar] [CrossRef]
- Lee, J.H.; Park, K.H.; Kim, H.T.; Seo, W.D.; Kim, J.Y.; Baek, I.Y.; Jang, D.S.; Ha, T.J. Identification, characterisation, and quantification of phenolic compounds in the antioxidant activity-containing fraction from the seeds of Korean perilla (Perilla frutescens) cultivars. Food Chem. 2013, 136, 843–852. [Google Scholar] [CrossRef] [PubMed]
- Jin, B.R.; Chung, K.S.; Cheon, S.Y.; Lee, M.; Hwang, S.; Noh, H.S.; Rhee, K.J.; An, H.J. Rosmarinic acid suppresses colonic inflammation in dextran sulphate sodium (DSS)-induced mice via dual inhibition of NF-κB and STAT3 activation. Sci. Rep. 2017, 7, 46252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ito, N.; Yabe, T.; Gamo, Y.; Nagai, T.; Oikawa, T.; Yamada, H.; Hanawa, T. Rosmarinic acid from Perillae Herba produces an antidepressant-like effect in mice through cell proliferation in the hippocampus. Biol. Pharm. Bull. 2008, 31, 1376–1380. [Google Scholar] [CrossRef] [PubMed]
- Wiederholt, M.; Sturm, A.; Lepple-Wienhues, A. Relaxation of trabecular meshwork and ciliary muscle by release of nitric oxide. Investig. Ophthalmol. Vis. Sci. 1994, 35, 2515–2520. [Google Scholar]
- Watanabe, N.; Tominaga, Y.; Mizutani, K.; Ogawa, T.; Tsunobuchi-Ushijima, H.; Gomi, Y. Inhibitory effects of amlexanox on carbachol-induced contractions of rabbit ciliary muscle and guinea-pig taenia caecum. J. Pharm. Pharmacol. 2000, 52, 1377–1385. [Google Scholar] [CrossRef] [PubMed]
- Mstsumoto, H. Delphinidin-3-rutinoside relaxes the bovine ciliary smooth muscle through activation of ETB receptor and NO/cGMP pathway. Exp. Eye Res. 2005, 80, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Choi, H.; Kim, M.R.; Oh, D.R.; Kim, Y.; Kang, H.; Jeong, K.I.; Ryu, G.C.; Pan, S.; Choi, C.Y. Amelioration of visual display terminal-induced ocular fatigue by aqueous extracts of perilla frutescens var. acuta. J. Food Nutr. Res. 2017, 5, 553–561. [Google Scholar] [CrossRef]
- Hiramoto, K.; Yamate, Y.; Orita, K.; Jikumaru, M.; Kasahara, E.; Sato, E.; Tamura, S.; Inoue, M. Prevention of scattered light-induced asthenopia and fatigue by a polarized filter. Photodermatol. Photoimmunol. Photomed. 2010, 26, 89–92. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Kim, A.Y.; Jo, A.; Choi, H.; Cho, S.S.; Choi, C. Development of User-Friendly Method to Distinguish subspecies of the Korean medicinal herb Perilla frutescens using multiplex-PCR. Molecules 2017, 22, 665. [Google Scholar] [CrossRef] [PubMed]
- Akagi, T.; Mandat, M.; Ooto, S.; Kageyama, R.; Yosbtmura, N.; Takabasbt, M. Otx2 homeobox gene induced photoreceptor-specific phenotypes in cells derived from adult iris and ciliary tissue. Invest. Opthalmol. Vis. Sci. 2004, 45, 4570–4575. [Google Scholar] [CrossRef] [PubMed]
- Huh, J.; Park, G.Y.; Im, J.M.; Jang, H.H.; Lee, I.; Moon, B.S. Effects of Hyeolbuchukeo-tang (Xiefuzhuyu-tang) on NO production in aortic vascular smooth muscle cells. J. Korean Orient. Med. 2003, 24, 166–178. [Google Scholar]
Sample Availability: Samples of the compounds are available from the authors. |







© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.; Kang, H.; Choi, H.; Jo, A.; Oh, D.-R.; Kim, Y.; Im, S.; Lee, S.-G.; Jeong, K.-I.; Ryu, G.-C.; et al. Aqueous Extract of Perilla frutescens var. acuta Relaxes the Ciliary Smooth Muscle by Increasing NO/cGMP Content In Vitro and In Vivo. Molecules 2018, 23, 1777. https://doi.org/10.3390/molecules23071777
Kim J, Kang H, Choi H, Jo A, Oh D-R, Kim Y, Im S, Lee S-G, Jeong K-I, Ryu G-C, et al. Aqueous Extract of Perilla frutescens var. acuta Relaxes the Ciliary Smooth Muscle by Increasing NO/cGMP Content In Vitro and In Vivo. Molecules. 2018; 23(7):1777. https://doi.org/10.3390/molecules23071777
Chicago/Turabian StyleKim, Jaeyong, Huwon Kang, Hakjoon Choi, Ara Jo, Dooi-Ri Oh, Yujin Kim, Sojeong Im, Seul-Gi Lee, Kyeong-In Jeong, Geun-Chang Ryu, and et al. 2018. "Aqueous Extract of Perilla frutescens var. acuta Relaxes the Ciliary Smooth Muscle by Increasing NO/cGMP Content In Vitro and In Vivo" Molecules 23, no. 7: 1777. https://doi.org/10.3390/molecules23071777