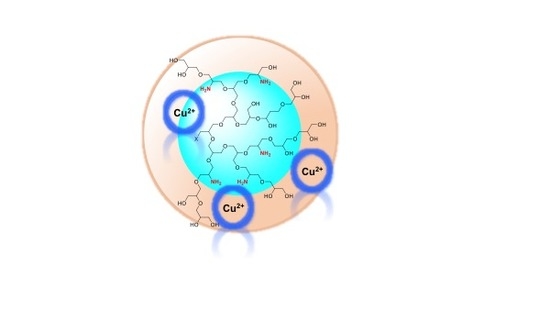
Hyperbranched Polyglycerol Derivatives as Prospective Copper Nanotransporter Candidates
Abstract
:1. Introduction
2. Results
2.1. Dialkylamine Modified hPG Synthesized through Nucleophilic Substitution Reaction
2.2. Selective Chemical Differentiation of Primary and Secondary Hydroxyl Groups of hPG: Cu-Binding Domains at hPG Core
2.3. Amide Coupling for the Synthesis of hPG-Bispicolylamide Derivatives
2.4. Reductive Amination Pathway towards the Synthesis of Mono-and Oligosaccharide Modified hPG-Amine
2.5. Attaching N-α-BOC-Histidine to Polyglycerolamine: BBB Targeted Nanocarriers
2.6. Synthesis of FITC-Labelled PG10-TMEDA System
2.7. Cu-Encapsulation by Synthesized hPG-Derived Nanocarriers: UV-Visible Spectroscopic Investigation
2.8. Thermodynamics of Cu-Ion Encapsulation by hPG Nanoconstructs: Isothermal Titration Calorimetry (ITC)
2.9. Surface Charge Characteristics of Cu-Loaded hPG Nanocarriers
2.10. Interaction of Cu-Ion Encapsulating Systems with Plasma Albumin
2.11. Cellular Toxicity and Uptake of Representative hPG-Derived Nanocarriers
3. Discussion
4. Experimental Section
4.1. Materials
4.2. UV-Vis Spectroscopy
4.3. Isothermal Titration Calorimetry (ITC)
4.4. Zeta Potential Measurement
4.5. Fluorescence Spectroscopic Studies
4.6. Synthesis of Core-Functionalized Polyglycerolamine; Ketalization of PG: Protection of Terminal Diols 4

4.7. Mesylation of Ketal Protected PG 5

4.8. Procedure for the Synthesis of Ketal Protected Polyglycerolazide 6

4.9. Deprotection of Ketal Protected Polyglycerol Azide 7

4.10. Synthesis of Core-Functionalized Polyglycerolamine 8

4.11. Core Functionalization of PG Acetal with N1,N1,N2-Trimethylethane-1,2-Diamine 9

4.12. Procedure for the Synthesis of PG-cNH2 Containing 6-((tert-butoxycarbonyl(pyridin-2-ylmethyl)-amino)methyl)nicotinic Acid 11

4.13. Procedure for the Synthesis of PG-cNH2 Containing 6-(((2-(tert-butoxycarbonyl(pyridin-2-ylmethyl)-amino)ethyl)(propyl)amino)methyl)nicotinic Acid 12

4.14. Procedure for the Synthesis of Maltose Modified PG Amine 14

4.15. Procedure for the Synthesis of N-Acetylglucosamine Modified PG Amine 15

4.16. Procedure for the Synthesis of Polyglycerolamine Containing N-α-BOC-Histidine 16

Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kim, B.-E.; Nevitt, T.; Thiele, D.J. Mechanism for copper acquisition, distribution and regulation. Nat. Chem. Biol. 2008, 4, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Ponka, P. Hereditary causes of disturbed iron homeostasis in the central nervous system. Ann. N. Y. Acad. Sci. 2004, 1012, 267–281. [Google Scholar] [CrossRef] [PubMed]
- Waggoner, D.J.; Bartnikas, T.B.; Gitlin, J.D. The role of copper in neurodegenerative disease. Neurobiol. Dis. 1999, 6, 221–230. [Google Scholar] [CrossRef] [PubMed]
- de Bie, P.; Muller, P.; Wijmenga, C.; Klomp, L.W. Molecular pathogenesis of wilson and menkes disease: Correlation of mutations with molecular defects and disease phenotypes. J. Med. Genet. 2007, 44, 673–688. [Google Scholar] [CrossRef] [PubMed]
- Adlard, P.A.; Bush, A.I. Metals in alzheimer’s disease. J. Alzheimer's Dis. 2006, 10, 145–163. [Google Scholar] [CrossRef]
- Kessler, H.; Bayer, T.A.; Bach, D.; Schneider-Axmann, T.; Supprian, T.; Herrmann, W.; Haber, M.; Multhaup, G.; Falkai, P.; Pajonk, F.G. Intake of copper has no effect on cognition in patients with mild alzheimer’s disease: A pilot phase 2 clinical trial. J. Neural Transm. 2008, 115, 1181–1187. [Google Scholar] [CrossRef] [PubMed]
- Phinney, A.L.; Drisaldi, B.; Schmidt, S.D.; Lugowski, S.; Coronado, V.; Liang, Y.; Horne, P.; Yang, J.; Sekoulidis, J.; Coomaraswamy, J.; et al. In vivo reduction of amyloid-beta by a mutant copper transporter. Proc. Natl. Acad. Sci. 2003, 100, 14193–14198. [Google Scholar] [CrossRef] [PubMed]
- Adlard, P.A.; Cherny, R.A.; Finkelstein, D.I.; Gautier, E.; Robb, E.; Cortes, M.; Volitakis, I.; Liu, X.; Smith, J.P.; Perez, K.; et al. Rapid restoration of cognition in alzheimer’s transgenic mice with 8-hydroxy quinoline analogs is associated with decreased interstitial abeta. Neuron 2008, 59, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Filomeni, G.; Cerchiaro, G.; Da Costa Ferreira, A.M.; De Martino, A.; Pedersen, J.Z.; Rotilio, G.; Ciriolo, M.R. Pro-apoptotic activity of novel isatin-schiff base copper(ii) complexes depends on oxidative stress induction and organelle-selective damage. J. Biol. Chem. 2007, 282, 12010–12021. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Friedman, J.E.; Angel, I.; Kozak, A.; Koh, J.Y. The lipophilic metal chelator dp-109 reduces amyloid pathology in brains of human beta-amyloid precursor protein transgenic mice. Neurobiol. Aging 2004, 25, 1315–1321. [Google Scholar] [CrossRef] [PubMed]
- Treiber, C.; Simons, A.; Strauss, M.; Hafner, M.; Cappai, R.; Bayer, T.A.; Multhaup, G. Clioquinol mediates copper uptake and counteracts copper efflux activities of the amyloid precursor protein of alzheimer’s disease. J. Biol. Chem. 2004, 279, 51958–51964. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, R.; Fehse, S.; Pant, K.; Nowag, S.; Stephan, H.; Haag, R.; Tzschucke, C.C. Polyglycerol-based copper chelators for the transport and release of copper ions in biological environments. Macromol. Biosci. 2016, 16, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Fehse, S.; Nowag, S.; Quadir, M.; Kim, K.S.; Haag, R.; Multhaup, G. Copper transport mediated by nanocarrier systems in a blood-brain barrier in vitro model. Biomacromolecules 2014, 15, 1910–1919. [Google Scholar] [CrossRef] [PubMed]
- Nowag, S.; Frangville, C.; Multhaup, G.; Marty, J.-D.; Mingotaud, C.; Haag, R. Biocompatible, hyperbranched nanocarriers for the transport and release of copper ions. J. Mater. Chem. B 2014, 2, 3915–3918. [Google Scholar] [CrossRef]
- Roller, S.; Zhou, H.; Haag, R. High-loading polyglycerol supported reagents for mitsunobu-and acylation-reaction and other polyglycerol derivatives. Mol. Divers. 2005, 9, 305–316. [Google Scholar] [CrossRef] [PubMed]
- Wooley, K.L.; Fréchet, J.M.J.; Hawker, C.J. Influence of shape on the reactivity and properties of dendritic, hyperbranched and linear aromatic polyesters. Polymer 1994, 35, 4489–4495. [Google Scholar] [CrossRef]
- Hawker, C.J.; Malmström, E.; Frank, C.W.; Kampf, J.P. Exact linear analogs of dendritic polyether macromolecules: design, synthesis, and unique properties. J. Am. Chem. Soc. 1997, 119, 9903–9904. [Google Scholar] [CrossRef]
- Mourey, T.H.; Turner, S.R.; Rubinstein, M.; Fréchet, J.M.J.; Hawker, C.J.; Wooley, K.L. Unique behavior of dendritic macromolecules: intrinsic viscosity of polyether dendrimers. Macromolecules 1992, 25, 2401–2406. [Google Scholar] [CrossRef]
- Wooley, K.L.; Hawker, C.J.; Pochan, J.M.; Fréchet, J.M.J. Physical properties of dendritic macromolecules: a study of glass transition temperature. Macromolecules 1993, 26, 1514–1519. [Google Scholar] [CrossRef]
- Hawker, C.J.; Farrington, P.J.; Mackay, M.E.; Wooley, K.L.; Fréchet, J.M.J. Molecular ball bearings: the unusual melt viscosity behavior of dendritic macromolecules. J. Am. Chem. Soc. 1995, 117, 4409–4410. [Google Scholar] [CrossRef]
- Grayson, S.M.; Fréchet, J.M.J. Convergent dendrons and dendrimers: from synthesis to applications. Chem. Rev. 2001, 101, 3819–3867. [Google Scholar] [CrossRef] [PubMed]
- Haag, R.; Stumbé, J.-F.; Sunder, A.; Frey, H.; Hebel, A. An approach to core—Shell-type architectures in hyperbranched polyglycerols by selective chemical differentiation. Macromolecules 2000, 33, 8158–8166. [Google Scholar] [CrossRef]
- Frey, H.; Haag, R. Dendritic polyglycerol: A new versatile biocompatible-material. J. Biotechnol. 2002, 90, 257–267. [Google Scholar] [CrossRef]
- Kurniasih, I.N.; Liang, H.; Kumar, S.; Mohr, A.; Sharma, S.K.; Rabe, J.P.; Haag, R. A bifunctional nanocarrier based on amphiphilic hyperbranched polyglycerol derivatives. J. Mater. Chem. B 2013, 1, 3569–3577. [Google Scholar] [CrossRef]
- Hanselmann, R.; Hölter, D.; Frey, H. Hyperbranched polymers prepared via the core-dilution/slow addition technique: computer simulation of molecular weight distribution and degree of branching. Macromolecules 1998, 31, 3790–3801. [Google Scholar] [CrossRef]
- Bröhmer, M.C.; Bannawarth, W. Forced complexation of nitrogen leading to a weakening of amide bonds: Application to a new linker for solid-phase chemistry. Eur. J. Org. Chem 2008, 2008, 4412–4415. [Google Scholar] [CrossRef]
- Sayre, L.M. Metal ion catalysis of amide hydrolysis. J. Am. Chem. Soc. 1986, 108, 1632–1635. [Google Scholar] [CrossRef]
- Polzin, G.M.; Burstyn, J.N. Synthetic Cu(II) and Ni(II) peptidases. Met. Ions Biol. Syst. 2001, 38, 103–144. [Google Scholar] [PubMed]
- Grant, K.B.; Kassari, M. Major advances in the hydrolysis of peptides and proteins by metal ions and complexes. Curr. Org. Chem. 2006, 10, 1035–1049. [Google Scholar] [CrossRef]
- Bhadra, D.; Yadav, A.K.; Bhadra, S.; Jain, N.K. Glycodendrimeric nanoparticulate carriers of primaquine phosphate for liver targeting. Int. J. Pharm. 2005, 295, 221–233. [Google Scholar] [CrossRef] [PubMed]
- Dutta, T.; Jain, N.K. Targeting potential and anti-HIV activity of lamivudine loaded mannosylated poly (propyleneimine) dendrimer. Biochim. Biophys. Acta 2007, 1770, 681–686. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.V.; Asthana, A.; Dutta, T.; Jain, N.K. Intracellular macrophage uptake of rifampicin loaded mannosylated dendrimers. J. Drug Target. 2006, 14, 546–556. [Google Scholar] [CrossRef] [PubMed]
- Arima, H.; Chihara, Y.; Arizono, M.; Yamashita, S.; Wada, K.; Hirayama, F.; Uekama, K. Enhancement of gene transfer activity mediated by mannosylated dendrimer/α-cyclodextrin conjugate (generation 3, G3). J. Control. Release 2006, 116, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Kunath, K.; von Harpe, A.; Fischer, D.; Kissel, T. Galactose-PEI–DNA complexes for targeted gene delivery: degree of substitution affects complex size and transfection efficiency. J. Control. Release 2003, 88, 159–172. [Google Scholar] [CrossRef]
- Baek, M.-K.; Roy, R. Synthesis and protein binding properties of T-antigen containing GlycoPAMAM dendrimers. Bioorg. Med. Chem. 2002, 10, 11–17. [Google Scholar] [CrossRef]
- Kensinger, R.D.; Yowler, B.C.; Benesi, A.J.; Schengrund, C.-L. Synthesis of novel, multivalent glycodendrimers as ligands for HIV-1 gp120. Bioconjug. Chem. 2004, 15, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Köhn, M.; Benito, J.; Mellet, C.O.; Lindhorst, T.K.; Fernández, J.M.G. Functional Evaluation of Carbohydrate-Centred Glycoclusters by Enzyme-Linked Lectin Assay: Ligands for Concanavalin A. ChemBioChem 2004, 5, 771–777. [Google Scholar] [CrossRef] [PubMed]
- Mangold, S.L.; Cloninger, M. Maximising multivalency effects in protein—Carbohydrate interactions. Org. Biomol. Chem. 2006, 4, 2458–2465. [Google Scholar] [CrossRef] [PubMed]
- Marotte, K.; Preville, C.; Sabin, C.; Moume-Pymbock, M.; Imberty, A.; Roy, R. Synthesis and binding properties of divalent and trivalent clusters of the Lewis a disaccharide moiety to Pseudomonas aeruginosa lectin PA-IIL. Org. Biomol. Chem. 2007, 5, 2953–2961. [Google Scholar] [CrossRef] [PubMed]
- Woller, E.K.; Walter, E.D.; Morgan, J.R.; Singel, D.J.; Cloninger, M.J. Altering the strength of lectin binding interactions and controlling the amount of lectin clustering using mannose/hydroxyl-functionalized dendrimers. J. Am. Chem. Soc. 2003, 125, 8820–8826. [Google Scholar] [CrossRef] [PubMed]
- Quadir, M.A.; Radowski, M.R.; Kratz, F.; Licha, K.; Hauff, P.; Haag, R. Dendritic multishell architectures for drug and dye transport. J. Control. Release 2008, 132, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Appelhans, D.; Komber, H.; Kirchner, R.; Seidel, J.; Huang, C.-H.; Voigt, D.; Kuckling, D.; Chang, F.-C.; Voit, B. Polypeptide-Shelled Poly (propylene imine) Dendrimers and Their Complexing Properties towards Copper (II) Ions. Macromol. Rapid Commun. 2005, 26, 586–593. [Google Scholar] [CrossRef]
- Baigude, H.; Katsuraya, K.; Okuyama, K.; Yachi, Y.; Sato, S.; Uryu, T. Synthesis of dicarboxylate oligosaccharide multilayer terminal functionality upon poly (lysine) dendrimer scaffolding. J. Polym. Sci. Part A Polym. Chem. 2002, 40, 3622–3633. [Google Scholar] [CrossRef]
- Klajnert, B.; Appelhans, D.; Komber, H.; Morgner, N.; Schwarz, S.; Richter, S.; Brutschy, B.; Ionov, M.; Tonkikh, A.K.; Bryszewska, M.; et al. The influence of densely organized maltose shells on the biological properties of poly (propylene imine) dendrimers: new effects dependent on hydrogen bonding. Chem. Eur. J. 2008, 14, 7030–7041. [Google Scholar] [CrossRef] [PubMed]
- Butt, A.M.; Jones, H.C. Effect of histamine and antagonists on electrical resistance across the blood-brain barrier in rat brain-surface microvessels. Brain Res. 1992, 569, 100–105. [Google Scholar] [CrossRef]
- Pardridge, W.M. Transport of small molecules through the blood-brain barrier: Biology and methodology. Adv. Drug Deliv. Rev. 1995, 15, 5–36. [Google Scholar] [CrossRef]
- Yamakami, J.; Sakurai, E.; Sakurada, T.; Maeda, K.; Hikichi, N. Stereoselective blood-brain barrier transport of histidine in rats. Brain Res. 1998, 812, 105–112. [Google Scholar] [CrossRef]
- Brackman, W.; Gaasbeek, C.J. Spectra and equilibria of some complexes containing the Cu2+ (phen)2 group. J. Inorg. Nucl. Chem. 1965, 27, 1793–1804. [Google Scholar] [CrossRef]
- Faye, G.H. The correlation of absorption spectra and structure of pseudo-octahedral and trigonal bipyramidal copper (II)-1,10-phenanthroline complexes in acetone solutions. Can. J. Chem. 1966, 44, 2165–2171. [Google Scholar] [CrossRef]
- James, B.R.; Parris, M.; Williams, R.J.P. Spectrophotometric and thermodynamic properties of some copper and iron complexes. J. Chem. Soc. 1961, 4630–4637. [Google Scholar] [CrossRef]
- Jorgensen, C.K. Comparative crystal field studies of some ligands and the lowest singlet state of paramagnetic nickel(II) complexes. Acta Chem. Scand. 1955, 9, 1362–1377. [Google Scholar] [CrossRef]
- Zhao, M.; Sun, L.; Crooks, R.M. Preparation of Cu nanoclusters within dendrimer templates. J. Am. Chem. Soc. 1998, 120, 4877–4878. [Google Scholar] [CrossRef]
- Esumi, K.; Suzuki, A.; Aihara, N.; Usui, K.; Torigoe, K. Preparation of gold colloids with UV irradiation using dendrimers as stabilizer. Langmuir 1998, 14, 3157–3159. [Google Scholar] [CrossRef]
- Crooks, R.M.; Zhao, M.; Sun, L.; Chechik, V.; Yeung, L.K. Dendrimer-encapsulated metal nanoparticles: Synthesis, characterization, and applications to catalysis. Acc. Chem. Res. 2001, 34, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Krämer, M.; Perignon, N.; Haag, R.; Marty, J.-D.; Thomann, R.; Lauth-de Viguerie, N.; Mingotaud, C. Water-soluble dendritic architectures with carbohydrate shells for the templation and stabilization of catalytically active metal nanoparticles. Macromolecules 2005, 38, 8308–8315. [Google Scholar] [CrossRef]
- Appelhans, D.; Komber, H.; Quadir, M.A.; Richter, S.; Schwarz, S.; van der Vlist, J.; Aigner, A.; Müller, M.; Loos, K.; Seidel, J.; et al. Hyperbranched pei with various oligosaccharide architectures: Synthesis, characterization, atp complexation, and cellular uptake properties. Biomacromolecules 2009, 10, 1114–1124. [Google Scholar] [CrossRef] [PubMed]
- Klajnert, B.; Bryszewska, M. Dendrimers: Properties and applications. Acta Biochim. Pol. 2001, 48, 199–208. [Google Scholar] [PubMed]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy, 2nd ed.; Kluwer Academic/Plenum Publishers: New York, NY, USA, 1999; pp. 238–255. [Google Scholar]
- Eftink, M.J. Fluorescence quenching reactions: Probing biological macromolecular structures. In Biophysical and Biochemical Aspects of Fluorescence Spectroscopy; Dewey, T.G., Ed.; Plenum Press: New York, NY, USA, 1991; pp. 1–41. [Google Scholar]
- Stern, O.; Volmer, M. Über die abklingzeit der fluoreszenz. Phys. Z. 1919, 20, 183–188. [Google Scholar]
- Cram, D.J.; Choi, H.J.; Bryant, J.A.; Knobler, C.B. Host-guest complexation. 62. Solvophobic and entropic driving forces for forming velcraplexes, which are 4-fold, lock-key dimers in organic media. J. Am. Chem. Soc. 1992, 114, 7748–7765. [Google Scholar] [CrossRef]
- Meissner, R.; Garcias, X.; Mecozzi, S.; Rebek, J.J. Synthesis and assembly of new molecular hosts: Solvation and the energetics of encapsulation. J. Am. Chem. Soc. 1997, 119, 77–85. [Google Scholar] [CrossRef]
- Peschke, W.; Schmidtchen, F.P. Incremental rigidification of a foldable anion host: Does it help in guest binding? Tetrahedron Lett. 1995, 36, 5155–5158. [Google Scholar] [CrossRef]
- Berger, M.; Schmidtchen, F.P. The binding of sulfate anions by guanidinium receptors is entropy-driven. Angew. Chem. Int. Ed. 1998, 37, 2694–2696. [Google Scholar] [CrossRef]
- Schneider, H.J. Binding mechanisms in supramolecular complexes. Angew. Chem. Int. Ed. 2009, 48, 3924–3977. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds can be made available upon re-synthesis. |










| Compoundno. | Compound Code | Structure |
|---|---|---|
 | ||
| 3a | PG10-DMA | R1 = R2 = -CH3 |
| 3b | PG10-DEA | R1 = R2 = -CH2-CH3 |
| 3c | PG10-DPA | R1 = R2 = -CH2-CH2-CH3 |
| 3d | PG10-DIPA | R1 = R2 = -CH(CH3)2 |
| 3e | PG10-DBA | R1 = R2 = -CH2-CH2-CH2-CH3 |
| 3f * | PG10-(TMEDA)x |  |
| X = Degree of functionalization, for 3f, X = 1.0 | ||
| 8 | PGm-cNH2 |  |
| 8a | m = 10 kDa | |
| 8b | m = 5 kDa | |
| 10 | PG10-cTMEDA |  |
| 11 | PG10-NF135 |  |
| 12 | PG10-KR455 |  |
| 14 | PG10-Mlt |  |
| 15 | PG10-GLNC |  |
| 16 | PG10-His |  |
| Compound | R1 = R2 | Yield (%) |
|---|---|---|
| 3a | CH3- | 60 |
| 3b | CH3CH2- | 86 |
| 3c | CH3CH2CH2- | 76 |
| 3d | (CH3)2-CH- | 56 |
| 3e | CH3CH2CH2CH2- | 68 |
| Compound No. | Nanocarriers | Mole of Cu2+/mol of Nanotransporter | |
|---|---|---|---|
| ITC | UV-Vis | ||
| 3f (50%) * | PG10-TMEDA0.5 | 31 | 36 |
| 3f (10%) * | PG10-TMEDA0.1 | 14 | 15 |
| 8a | PG10-cNH2 | 26 | 31 |
| 8b | PG5-cNH2 | 10 | 17 |
| 11 | PG10-NF 135 | 45 | 40 |
| 12 | PG10-KR 455 | 55 | 48 |
| 14 | PG10-Mlt | N/D | 40 |
| 15 | PG10-GLNC | N/D | 42 |
| 16 | PG10-His | 35 | N/D |
| Compound No. | Nanocarriers | Zeta Potential (mV) |
|---|---|---|
| 11 | PG10-NF 135 | (+) 36.0 ± 1.0 |
| 12 | PG10-KR 455 | (+) 38.5 ± 0.5 |
| 3a | PG10-DMA | (+) 28.0 ± 0.5 |
| 14 | PG10-Mlt | (−) 1.5 ± 1.0 |
| 15 | PG10-GLNC | (+) 5.0 ± 0.5 |
| 16 | PG10-His | (+) 8.5 ± 1.0 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quadir, M.; Fehse, S.; Multhaup, G.; Haag, R. Hyperbranched Polyglycerol Derivatives as Prospective Copper Nanotransporter Candidates. Molecules 2018, 23, 1281. https://doi.org/10.3390/molecules23061281
Quadir M, Fehse S, Multhaup G, Haag R. Hyperbranched Polyglycerol Derivatives as Prospective Copper Nanotransporter Candidates. Molecules. 2018; 23(6):1281. https://doi.org/10.3390/molecules23061281
Chicago/Turabian StyleQuadir, Mohiuddin, Susanne Fehse, Gerhard Multhaup, and Rainer Haag. 2018. "Hyperbranched Polyglycerol Derivatives as Prospective Copper Nanotransporter Candidates" Molecules 23, no. 6: 1281. https://doi.org/10.3390/molecules23061281