Ginsenoside Rg3 Prevents Oxidative Stress-Induced Astrocytic Senescence and Ameliorates Senescence Paracrine Effects on Glioblastoma
Abstract
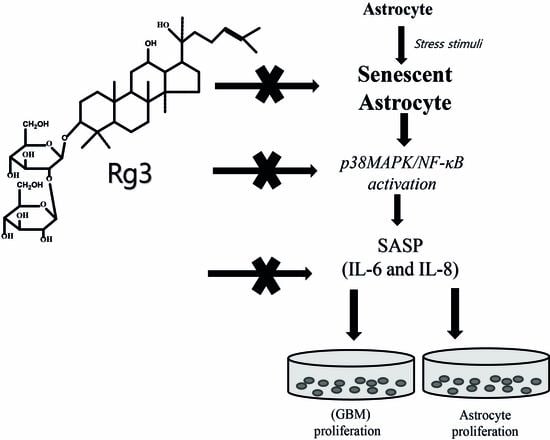
:1. Introduction
2. Results
2.1. Selected Ginsenosides Prevent Astrocytic Senescence and Decrease SASP
2.2. Ginsenoside Rg3 Decreases SASP by Suppressing NF-κB Nuclear Translocation and p-38 Activation
2.3. Selected Ginsenosides Do Not Reverse Cellular Senescence
2.4. Ginsenoside Rg3 Decreases the Ability of Senescent Astrocytes to Inhibit Normal Cell Growth
2.5. Oxidative Stress-Induced Astrocytic Senescence Secrets High Level of IL-8
2.6. Ginsenoside Rg3 Decreases Paracrine Effects of Senescent Astrocytic Cells on Glioblastomas
3. Discussion
4. Materials and Methods
4.1. Cells and Culture Conditions
4.2. Reagents
4.3. Senescence Induction
4.4. SA-β-Gal Staining
4.5. Preparation of Conditioned Medium (CM), Antibody Array Test and Quantification of IL-6 and IL-8 Secretion
4.6. Cell Viability Assay
4.7. Immunoblotting
4.8. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The hallmarks of aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-H.; Hales, C.N.; Ozanne, S.E. DNA damage, cellular senescence and organismal ageing: Causal or correlative? Nucleic Acids Res. 2007, 35, 7417–7428. [Google Scholar] [CrossRef] [PubMed]
- Davalli, P.; Mitic, T.; Caporali, A.; Lauriola, A.; D’Arca, D. ROS, cell senescence, and novel molecular mechanisms in aging and age-related diseases. Oxid. Med. Cell. Longev. 2016, 2016, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Sohal, R.S.; Dubey, A. Mitochondrial oxidative damage, hydrogen peroxide release, and aging. Free Radic. Biol. Med. 1994, 16, 621–626. [Google Scholar] [CrossRef]
- Bernadotte, A.; Mikhelson, V.M.; Spivak, I.M. Markers of cellular senescence. Telomere shortening as a marker of cellular senescence. Aging (Albany NY) 2016, 8, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Floyd, R.A.; Hensley, K. Oxidative stress in brain aging: Implications for therapeutics of neurodegenerative diseases. Neurobiol. Aging 2002, 23, 795–807. [Google Scholar] [CrossRef]
- Ransom, B.R.; Ransom, C.B. Astrocytes: multitalented stars of the central nervous system. Methods Mol. Biol. 2012, 8, 3–7. [Google Scholar]
- Nichols, N.R.; Day, J.R.; Laping, N.J.; Johnson, S.A.; Finch, C.E. GFAP mRNA increases with age in rat and human brain. Neurobiol. Aging 1993, 14, 421–429. [Google Scholar] [CrossRef]
- Menet, V.; Ribotta, G.Y.; Sandillon, F.; Privat, A. GFAP null astrocytes are a favorable substrate for neuronal survival and neurite growth. Glia 2000, 31, 267–272. [Google Scholar] [CrossRef]
- Campuzano, O.; Castillo-Ruiz, M.; Acarin, L.; Castellano, B.; Gonzalez, B. Increased levels of proinflammatory cytokines in the aged rat brain attenuate injury-induced cytokine response after excitotoxic damage. J. Neurosci. Res. 2009, 87, 2484–2497. [Google Scholar] [CrossRef] [PubMed]
- Bitto, A.; Sell, C.; Crowe, E.; Lorenzini, A.; Malaguti, M.; Hrelia, S.; Torres, C. Stress-induced senescence in human and rodent astrocytes. Exp. Cell Res. 2010, 316, 2961–2968. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Cho, T.; Jantaratnotai, N.; Wang, Y.T.; McGeer, E.; McGeer, P.L. Depletion of GSH in glial cells induces neurotoxicity: Relevance to aging and degenerative neurological diseases. FASEB J. 2010, 24, 2533–2545. [Google Scholar] [CrossRef] [PubMed]
- Pertusa, M.; García-Matas, S.; Rodríguez-Farré, E.; Sanfeliu, C.; Cristòfol, R. Astrocytes aged in vitro show a decreased neuroprotective capacity. J. Neurochem. 2007, 101, 794–805. [Google Scholar] [CrossRef] [PubMed]
- Bhat, R.; Crowe, E.P.; Bitto, A.; Moh, M.; Katsetos, C.D.; Garcia, F.U.; Johnson, F.B.; Trojanowski, J.Q.; Sell, C.; Torres, C. Astrocyte senescence as a component of Alzheimer’s disease. PLoS ONE 2012, 7. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.-A.; Eun, J.-S.; Oh, K.-W. Therapeutic effects of ginseng on psychotic disorders. J. Ginseng Res. 2007, 31, 117–126. [Google Scholar]
- Zhang, X.; Wang, Y.; Ma, C.; Yan, Y.; Yang, Y.; Wang, X.; Rausch, W.-D. Ginsenoside Rd and ginsenoside Re offer neuroprotection in a novel model of Parkinson’s disease. Am. J. Neurodegener. Dis. 2016, 5, 52–61. [Google Scholar] [PubMed]
- Wu, J.; Jeong, H.K.; Bulin, S.E.; Kwon, S.W.; Park, J.H.; Bezprozvanny, I. Ginsenosides protect striatal neurons in a cellular model of Huntington's disease. J. Neurosci. Res. 2009, 87, 1904–1912. [Google Scholar] [CrossRef] [PubMed]
- González-Burgos, E.; Fernandez-Moriano, C.; Gómez-Serranillos, M.P. Potential neuroprotective activity of Ginseng in Parkinson’s disease: A review. J. Neuroimmune Pharmacol. 2015, 10, 14–29. [Google Scholar] [CrossRef] [PubMed]
- Chien, Y.; Scuoppo, C.; Wang, X.; Fang, X.; Balgley, B.; Bolden, J.E.; Premsrirut, P.; Luo, W.; Chicas, A.; Lee, C.S. Control of the senescence-associated secretory phenotype by NF-κB promotes senescence and enhances chemosensitivity. Genes Dev. 2011, 25, 2125–2136. [Google Scholar] [CrossRef] [PubMed]
- Freund, A.; Patil, C.K.; Campisi, J. p38MAPK is a novel DNA damage response-independent regulator of the senescence-associated secretory phenotype. EMBO J. 2011, 30, 1536–1548. [Google Scholar] [CrossRef] [PubMed]
- Acosta, J.C.; O'Loghlen, A.; Banito, A.; Guijarro, M.V.; Augert, A.; Raguz, S.; Fumagalli, M.; Da Costa, M.; Brown, C.; Popov, N. Chemokine signaling via the CXCR2 receptor reinforces senescence. Cell 2008, 133, 1006–1018. [Google Scholar] [CrossRef] [PubMed]
- Krtolica, A.; Parrinello, S.; Lockett, S.; Desprez, P.-Y.; Campisi, J. Senescent fibroblasts promote epithelial cell growth and tumorigenesis: A link between cancer and aging. Proc. Natl. Acad. Sci. USA 2001, 98, 12072–12077. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Hornsby, P.J. Senescent human fibroblasts increase the early growth of xenograft tumors via matrix metalloproteinase secretion. Cancer Res. 2007, 67, 3117–3126. [Google Scholar] [CrossRef] [PubMed]
- Olney, J.W.; Farber, N.B.; Spitznagel, E.; Robins, L.N. Increasing brain tumor rates: Is there a link to aspartame? J. Neuropathol. Exp. Neurol. 1996, 55, 1115–1123. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Morgan, T.E.; Rozovsky, I.; Finch, C.E. Aging and glial responses to lipopolysaccharide in vitro: Greater induction of IL-1 and IL-6, but smaller induction of neurotoxicity. Exp. Neurol. 2003, 182, 135–141. [Google Scholar] [CrossRef]
- Liu, Q.; Li, G.; Li, R.; He, Q.; Deng, L.; Zhang, C.; Zhang, J. IL-6 promotion of glioblastoma cell invasion and angiogenesis in U251 and T98G cell lines. J. Neurooncol. 2010, 100, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Kim, S.-H.; Jeon, H.-M.; Beck, S.; Sohn, Y.-W.; Yin, J.; Kim, J.-K.; Lim, Y.C.; Lee, J.-H.; Kim, S.-H. Interferon regulatory factor 7 regulates glioma stem cells via interleukin-6 and Notch signalling. Brain 2012, 135, 1055–1069. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Wang, Q.; Giang, A.; Cheng, C.; Soo, C.; Wang, C.-Y.; Liau, L.M.; Chiu, R. Knockdown of CypA inhibits interleukin-8 (IL-8) and IL-8-mediated proliferation and tumor growth of glioblastoma cells through down-regulated NF-κB. J. Neurooncol. 2011, 101, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Pasi, F.; Facoetti, A.; Nano, R. IL-8 and IL-6 bystander signalling in human glioblastoma cells exposed to gamma radiation. Anticancer Res. 2010, 30, 2769–2772. [Google Scholar] [PubMed]
- Bruyère, C.; Mijatovic, T.; Lonez, C.; Spiegl-Kreinecker, S.; Berger, W.; Kast, R.E.; Ruysschaert, J.M.; Kiss, R.; Lefranc, F. Temozolomide-induced modification of the CXC chemokine network in experimental gliomas. Int. J. Oncol. 2012, 38, 1453–1464. [Google Scholar]
- Kim, S.N.; Ha, Y.W.; Shin, H.; Son, S.H.; Wu, S.J.; Kim, Y.S. Simultaneous quantification of 14 ginsenosides in Panax ginseng CA Meyer (Korean red ginseng) by HPLC-ELSD and its application to quality control. J. Pharm. Biomed. Anal. 2007, 45, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Kang, X.; Zhao, W. Antiangiogenic effect of low-dose cyclophosphamide combined with ginsenoside Rg3 on Lewis lung carcinoma. Biochem. Biophys. Res. Commun. 2006, 342, 824–828. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Kang, X.; Yang, B.; Wang, J.; Yang, F. Antiangiogenic effect of capecitabine combined with ginsenoside Rg3 on breast cancer in mice. Cancer Biother. Radiopharm. 2008, 23, 647–654. [Google Scholar] [CrossRef] [PubMed]
- Gewirtz, D.A.; Holt, S.E.; Elmore, L.W. Accelerated senescence: An emerging role in tumor cell response to chemotherapy and radiation. Biochem. Pharmacol. 2008, 76, 947–957. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Liu, L.; Yu, Y.; Chen, B.; Tang, C.; Li, X. Antitumor effects of ginsenoside Rg3 on human hepatocellular carcinoma cells. Mol. Med. Rep. 2012, 5, 1295–1298. [Google Scholar]
- Chen, J.; Peng, H.; Ou-Yang, X.; He, X. Research on the antitumor effect of ginsenoside Rg3 in B16 melanoma cells. Melanoma Res. 2008, 18, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Shan, X.; Aziz, F.; Tian, L.L.; Wang, X.Q.; Yan, Q.; Liu, J.W. Ginsenoside Rg3-induced EGFR/MAPK pathway deactivation inhibits melanoma cell proliferation by decreasing FUT4/LeY expression. Int. J. Oncol. 2015, 46, 1667–1676. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Yu, Y.; Wang, L.; Wu, B.; Xia, L.; Feng, F.; Ling, Z.; Wang, S. Additive antiangiogenesis effect of ginsenoside Rg3 with low-dose metronomic temozolomide on rat glioma cells both in vivo and in vitro. J. Exp. Clin. Cancer Res. 2016, 35. [Google Scholar] [CrossRef] [PubMed]
- Johnsen, K.B.; Gudbergsson, J.M.; Skov, M.N.; Pilgaard, L.; Moos, T.; Duroux, M. A comprehensive overview of exosomes as drug delivery vehicles—endogenous nanocarriers for targeted cancer therapy. BBA Rev. Cancer 2014, 1846, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Zhuang, X.; Xiang, X.; Liu, Y.; Zhang, S.; Liu, C.; Barnes, S.; Grizzle, W.; Miller, D.; Zhang, H.G. A novel nanoparticle drug delivery system: The anti-inflammatory activity of curcumin is enhanced when encapsulated in exosomes. Mol. Ther. 2010, 18, 1606–1614. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Erviti, L.; Seow, Y.; Yin, H.; Betts, C.; Lakhal, S.; Wood, M.J. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat. Biotechnol. 2011, 29, 341–345. [Google Scholar] [CrossRef] [PubMed]
- André, F.; Chaput, N.; Schartz, N.E.; Flament, C.; Aubert, N.; Bernard, J.; Lemonnier, F.; Raposo, G.; Escudier, B.; Hsu, D.H. Exosomes as potent cell-free peptide-based vaccine. I. Dendritic cell-derived exosomes transfer functional MHC class I/peptide complexes to dendritic cells. J. Immunol. 2004, 172, 2126–2136. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Li, S.; Song, J.; Ji, T.; Zhu, M.; Anderson, G.J.; Wei, J.; Nie, G. A doxorubicin delivery platform using engineered natural membrane vesicle exosomes for targeted tumor therapy. Biomaterials 2014, 35, 2383–2390. [Google Scholar] [CrossRef] [PubMed]
- Choi, C.; Xu, X.; Oh, J.-W.; Lee, S.J.; Gillespie, G.Y.; Park, H.; Jo, H.; Benveniste, E.N. Fas-induced expression of chemokines in human glioma cells. Cancer Res. 2001, 61, 3084–3091. [Google Scholar] [PubMed]
- Choi, C.; Kutsch, O.; Park, J.; Zhou, T.; Seol, D.-W.; Benveniste, E.N. Tumor necrosis factor-related apoptosis-inducing ligand induces caspase-dependent interleukin-8 expression and apoptosis in human astroglioma cells. Mol. Cell. Biol. 2002, 22, 724–736. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds Ginsenoside Rg3 are available from the authors. |







© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hou, J.; Kim, S.; Sung, C.; Choi, C. Ginsenoside Rg3 Prevents Oxidative Stress-Induced Astrocytic Senescence and Ameliorates Senescence Paracrine Effects on Glioblastoma. Molecules 2017, 22, 1516. https://doi.org/10.3390/molecules22091516
Hou J, Kim S, Sung C, Choi C. Ginsenoside Rg3 Prevents Oxidative Stress-Induced Astrocytic Senescence and Ameliorates Senescence Paracrine Effects on Glioblastoma. Molecules. 2017; 22(9):1516. https://doi.org/10.3390/molecules22091516
Chicago/Turabian StyleHou, Jingang, Sunchang Kim, Changkeun Sung, and Chulhee Choi. 2017. "Ginsenoside Rg3 Prevents Oxidative Stress-Induced Astrocytic Senescence and Ameliorates Senescence Paracrine Effects on Glioblastoma" Molecules 22, no. 9: 1516. https://doi.org/10.3390/molecules22091516