FeCl3∙6H2O/TMSBr-Catalyzed Rapid Synthesis of Dihydropyrimidinones and Dihydropyrimidinethiones under Microwave Irradiation
Abstract
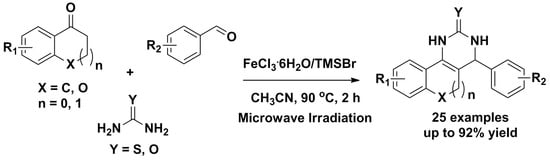
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. General Information
3.2. General Procedure for the Synthesis of 3,4-Dihydropyrimidin-2(1H)-thiones (4)
3.3. General Procedure for the Synthesis of 3,4-Dihydropyrimidin-2(1H)-ones (6)
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sari, O.; Roy, V.; Métifiot, M.; Marchand, C.; Pommier, Y.; Bourg, S.; Bonnet, P.; Schinazi, R.F.; Agrofoglio, L.A. Synthesis of dihydropyrimidine α,γ-diketobutanoic acid derivatives targeting HIV integrase. Eur. J. Med. Chem. 2015, 104, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Lacotte, P.; Puente, C.; Ambroise, Y. Synthesis and evaluation of 3,4-dihydropyrimidin-2(1H)-ones as sodium iodide symporter inhibitors. ChemMedChem 2013, 8, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Park, C.; Ok, T.; So, W.; Jo, M.; Seo, M.; Kim, Y.; Sohn, J.-H.; Park, Y.; Ju, M.K.; et al. Discovery of 3,4-dihydropyrimidin-2(1H)-ones with inhibitory activity against HIV-1 replication. Bioorg. Med. Chem. Lett. 2012, 22, 2119–2124. [Google Scholar] [CrossRef] [PubMed]
- Gijsen, H.J.M.; Berthelot, D.; Cleyn, M.A.J.D.; Geuens, I.; Brône, B.; Mercken, M. Tricyclic 3,4-dihydropyrimidine-2-thione derivatives as potent TRPA1 antagonists. Bioorg. Med. Chem. Lett. 2012, 22, 797–800. [Google Scholar] [CrossRef] [PubMed]
- Lokwani, D.; Azad, R.; Sarkate, A.; Reddanna, P.; Shinde, D. Structure based library design (SBLD) for new 1,4-dihydropyrimidine scaffold as simultaneous COX-1/COX-2 and 5-LOX inhibitors. Bioorg. Med. Chem. 2015, 23, 4533–4543. [Google Scholar] [CrossRef] [PubMed]
- Bhosle, M.R.; Deshmukh, A.R.; Pal, S.; Srivastava, A.K.; Mane, R.A. Synthesis of new thiazolylmethoxyphenyl pyrimidines and antihyperglycemic evaluation of the pyrimidines, analogues isoxazolines and pyrazolines. Bioorg. Med. Chem. Lett. 2015, 25, 2442–2446. [Google Scholar] [CrossRef] [PubMed]
- Mokale, S.N.; Shinde, S.S.; Elgire, R.D.; Sangshetti, J.N.; Shinde, D.B. Synthesis and anti-inflammatory activity of some 3-(4,6-disubtituted-2-thioxo-1,2,3,4-tetrahydropyrimidin-5-yl) propanoic acid derivatives. Bioorg. Med. Chem. Lett. 2010, 20, 4424–4426. [Google Scholar] [CrossRef] [PubMed]
- Bahekar, S.S.; Shinde, D.B. Synthesis and anti-inflammatory activity of some [4,6-(4-substituted aryl)-2-thioxo-1,2,3,4-tetrahydro-pyrimidin-5-yl]-acetic acid derivatives. Bioorg. Med. Chem. Lett. 2004, 14, 1733–1736. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Ding, X.; Chen, T.; Chen, L.; Liu, F.; Jia, X.; Lu, X.; Shen, X.; Che, K.; Jian, H.; et al. Design, synthesis, and interaction study of quinazoline-2(1H)-thione derivatives as novel potential Bcl-xL inhibitors. J. Med. Chem. 2010, 53, 3465–3479. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Chen, C.; Liu, H.; Zheng, L.; Tong, Y.; Qu, D.; Han, S. Biological evaluation of halogenated thiazolo [3,2-a] pyrimidin-3-one carboxylic acid derivatives targeting the YycG histidine kinase. Eur. J. Med. Chem. 2014, 87, 500–507. [Google Scholar] [CrossRef] [PubMed]
- Arnold, M.A.; Day, K.A.; Durón, S.G.; Gin, D.Y. Total synthesis of (+)-Batzelladine A and (−)-Batzelladine D via [4+2]-annulation of vinyl carbodiimides with N-alkyl imines. J. Am. Chem. Soc. 2006, 128, 13255–13260. [Google Scholar] [CrossRef] [PubMed]
- Shimokawa, J.; Ishiwata, T.; Shirai, K.; Koshino, H.; Tanatani, A.; Nakata, T.; Hashimoto, Y.; Nagasawa, K. Total synthesis of (+)-batzelladine A and (−)-batzelladine D, and identification of their target protein. Chem. Eur. J. 2005, 11, 6878–6888. [Google Scholar] [CrossRef] [PubMed]
- Patil, A.D.; Freyer, A.J.; Taylor, P.B.; Carté, B.; Zuber, G.; Johnson, R.K.; Faulkner, D.J. Batzelladines F−I, novel alkaloids from the sponge Batzella. sp.: Inducers of p56lck-CD4 dissociation. J. Org. Chem. 1997, 62, 1814–1819. [Google Scholar] [CrossRef]
- Patil, A.D.; Kumar, N.V.; Kokke, W.C.; Bean, M.F.; Freyer, A.J.; Brosse, C.D.; Mai, S.; Truneh, A.; Faulkner, D.J.; Carte, B.; et al. Novel alkaloids from the sponge Batzella. sp.: Inhibitors of HIV gp120-human CD4 binding. J. Org. Chem. 1995, 60, 1182–1188. [Google Scholar] [CrossRef]
- Bordner, J.; Thiessen, W.E.; Bates, H.A.; Rapoport, H. Structure of a crystalline derivative of saxitoxin. Structure of saxitoxin. J. Am. Chem. Soc. 1975, 97, 6008–6012. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.; Singh, K.; Wan, B.; Franzblau, S.; Chibale, K.; Balzarini, J. Facile transformation of Biginelli pyrimidin-2(1H)-ones to pyrimidines. In vitro evaluation as inhibitors of Mycobacterium tuberculosis and modulators of cytostatic activity. Eur. J. Med. Chem. 2011, 46, 2290–2294. [Google Scholar] [CrossRef] [PubMed]
- Amr, A.G.; Mohamed, A.M.; Mohamed, S.F.; Abdel-Hafez, N.A.; Hammam, A.-F. Anticancer activities of some newly synthesized pyridine, pyrane, and pyrimidine derivatives. Bioorg. Med. Chem. 2006, 14, 5481–5488. [Google Scholar] [CrossRef] [PubMed]
- Kappe, C.O. Biologically active dihydropyrimidones of the Biginelli-type—A literature survey. Eur. J. Med. Chem. 2000, 35, 1043–1052. [Google Scholar] [CrossRef]
- Kaan, H.Y.; Ulaganathan, V.; Rath, O.; Prokopcová, H.; Dallinger, D.; Kappe, C.O.; Kozielski, F. Structural basis for inhibition of Eg5 by dihydropyrimidines: Stereoselectivity of antimitotic inhibitors enastron, dimethylenastron and fluorastrol. J. Med. Chem. 2010, 53, 5676–5683. [Google Scholar] [CrossRef] [PubMed]
- Sadanandam, Y.S.; Shetty, M.M.; Diwan, P.V. Synthesis and biological evaluation of new 3,4-dihydro-6-methyl-5-N-methyl-carbamoyl-4-(substituted phenyl)-2(1H)pyrimidinones and pyrimidinethiones. Eur. J. Med. Chem. 1992, 27, 87–92. [Google Scholar] [CrossRef]
- Hurst, E.W.; Hull, R. Two new synthetic substances active against viruses of the psittacosis-lymphogranuloma-trachoma group. J. Med. Chem. 1960, 3, 215–229. [Google Scholar] [CrossRef]
- Rovnyak, G.C.; Atwal, K.S.; Hedberg, A.; Kimball, S.D.; Moreland, S.; Gougoutas, J.Z.; O’Reilly, B.C.; Schwartz, J.; Malley, M.F. Dihydropyrimidine calcium channel blockers. 4. Basic 3-substituted-4-aryl-1,4-dihydropyrimidine-5-carboxylic acid esters. Potent antihypertensive agents. J. Med. Chem. 1992, 35, 3254–3263. [Google Scholar] [CrossRef] [PubMed]
- Atwal, K.S.; Swanson, B.N.; Unger, S.E.; Floyd, D.M.; Moreland, S.; Hedberg, A.; O’Reilly, B.C. Dihydropyrimidine calcium channel blockers. 3. 3-Carbamoyl-4-aryl-1,2,3,4-tetrahydro-6-methyl-5-pyrimidinecarboxylic acid esters as orally effective antihypertensive agents. J. Med. Chem. 1991, 34, 806–811. [Google Scholar] [CrossRef] [PubMed]
- Rovnyak, G.C.; Kimball, S.D.; Beyer, B.; Cucinotta, G.; DiMarco, J.D.; Gougoutas, J.Z.; Hedberg, A.; Malley, M.F.; McCarthy, J.P.; Zhang, R.; et al. Calcium entry blockers and activators: Conformational and structural determinants of dihydropyrimidine calcium channel modulators. J. Med. Chem. 1995, 38, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Kappe, C.O. 4-Aryldihydropyrimidines via the Biginelli condensation: Aza-analogs of nifedipine-type calcium channel modulators. Molecules 1998, 3, 1–9. [Google Scholar] [CrossRef]
- Atwal, K.S.; Rovnyak, G.C.; Kimball, S.D.; Floyd, D.M.; Moreland, S.; Swanson, B.N.; Gougoutas, J.Z.; Schwartz, J.; Smillie, K.M.; Malley, M.F. Dihydropyrimidine calcium channel blockers. II. 3-Substituted-4-aryl-1,4-dihydro-6-methyl-5-pyrimidinecarboxylic acid esters as potent mimics of dihydropyridines. J. Med. Chem. 1990, 33, 2629–2635. [Google Scholar] [CrossRef] [PubMed]
- Jauk, B.; Pernat, T.; Kappe, C.O. Design and synthesis of a conformationally rigid mimic of the dihydropyrimidine calcium channel modulator SQ 32,926. Molecules 2000, 5, 227–239. [Google Scholar] [CrossRef]
- Lagu, B.; Tian, D.; Nagarathnam, D.; Marzabadi, M.R.; Wong, W.C.; Miao, S.W.; Zhang, F.-Q.; Sun, W.-Y.; Chiu, G.; Fang, J.; et al. Design and synthesis of novel α1a adrenoceptor-selective antagonists. 3. Approaches to eliminate opioid agonist metabolites by using substituted phenylpiperazine side chains. J. Med. Chem. 1999, 42, 4794–4803. [Google Scholar] [CrossRef] [PubMed]
- Nagarathnam, D.; Miao, S.-W.; Lagu, B.; Chiu, G.; Fang, J.; Dhar, T.G.M.; Zhang, J.; Tyagarajan, S.; Marzabadi, M.R.; Zhang, F.-Q.; et al. Design and synthesis of novel α1a adrenoceptor-selective antagonists. 1. Structure−activity relationship in dihydropyrimidinones. J. Med. Chem. 1999, 42, 4764–4777. [Google Scholar] [CrossRef] [PubMed]
- Cech, D.; Hein, L.; Wattke, R.; Janta-Lipinski, M.V.; Otto, A.; Langen, P. Synthesis of substituted 5-fluoro-5, 6-dihydropyrimidines. Nucleic Acid. Res. 1975, 2, 2177–2182. [Google Scholar] [CrossRef] [PubMed]
- Biginelli, P. The urea-aldehyde derivatives of acetoacetic esters. Gazz. Chim. Ital. 1893, 23, 360–416. [Google Scholar]
- Lu, J.; Bai, Y.-J.; Wang, Z.-J.; Yang, B.-Q.; Ma, H.-R. One-pot synthesis of 3,4-dihydropyrimidin-2(1H)-ones using lanthanum chloride as a catalyst. Tetrahedron Lett. 2000, 41, 9075–9078. [Google Scholar] [CrossRef]
- Ma, Y.; Qian, C.-T.; Wang, L.-M.; Yang, M. Lanthanide triflate catalyzed Biginelli reaction. One-pot synthesis of dihydropyrimidinones under solvent-free conditions. J. Org. Chem. 2000, 65, 3864–3868. [Google Scholar] [CrossRef] [PubMed]
- Reddy, C.V.; Mahesh, M.; Raju, P.V.K.; Babu, T.R.; Reddy, V.V.N. Zirconium(IV) chloride catalyzed one-pot synthesis of 3,4-dihydropyrimidin-2(1H)-ones. Tetrahedron Lett. 2002, 43, 2657–2659. [Google Scholar] [CrossRef]
- Fu, N.-Y.; Yuan, Y.-F.; Cao, Z.; Wang, S.-W.; Wang, J.-T.; Peppe, C. Indium(III) bromide-catalyzed preparation of dihydropyrimidinones: Improved protocol conditions for the Biginelli reaction. Tetrahedron 2002, 58, 4801–4807. [Google Scholar] [CrossRef]
- Varala, R.; Alam, M.M.; Adapa, S.R. Bismuth triflate catalyzed one-pot synthesis of 3,4-dihydropyrimidin-2 (1H)-ones: An improved protocol for the Biginelli reaction. Synlett 2003, 67–70. [Google Scholar] [CrossRef]
- Yadav, J.S.; Reedy, B.V.S.; Srinivas, R.; Venugopal, C.; Ramalingam, T. LiClO4-catalyzed one-pot synthesis of dihydropyrimidinones: An improved protocol for Biginelli reaction. Synthesis 2001, 1341–1345. [Google Scholar] [CrossRef]
- Dondoni, A.; Massi, A.; Minghini, E.; Sabbatini, S.; Bertolasi, V. Model studies toward the synthesis of dihydropyrimidinyl and pyridyl α-amino acids via three-component Biginelli and Hantzsch cyclocondensations. J. Org. Chem. 2003, 68, 6172–6183. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Bai, Y.-J.; Guo, Y.-H.; Wang, Z.-J.; Ma, H.-R. CoCl2·6H2O or LaCl3·7H2O catalyzed Biginelli reaction. one-pot synthesis of 3,4-dihydropyrimidin-2(1H)-ones. Chin. J. Chem. 2002, 20, 681–687. [Google Scholar] [CrossRef]
- Labrie, P. NiCl2 and NiCl2·6H2O: A very useful mild lewis acid in organic synthesis. Synlett 2003, 279–280. [Google Scholar] [CrossRef]
- Russowsky, D.; Lopes, F.A.; Silva, V.S.S.; Canto, K.F.S.; D’Oca, M.G.M.; Godoi, M.N. Multicomponent Biginelli’s synthesis of 3,4-dihydropyrimidin-2(1H)-ones promoted by SnCl2·2H2O. J. Braz. Chem. Soc. 2004, 15, 165–169. [Google Scholar] [CrossRef]
- Bose, D.S.; Fatima, L.; Mereyala, H.B. Green chemistry approaches to the synthesis of 5-alkoxycarbonyl-4-aryl-3,4-dihydropyrimidin-2(1H)-ones by a three-component coupling of one-pot condensation reaction: Comparison of ethanol, water, and solvent-free conditions. J. Org. Chem. 2003, 68, 587–590. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.A.; Kasthuraiah, M.; Reddy, C.S.; Reddy, C.D. Mn(OAc)3·2H2O-mediated three-component, one-pot, condensation reaction: An efficient synthesis of 4-aryl-substituted 3,4-dihydropyrimidin-2-ones. Tetrahedron Lett. 2001, 42, 7873–7875. [Google Scholar] [CrossRef]
- Mirza-Aghayan, M.; Moradi, A.; Bolourtchian, M. A novel and efficient one-pot method to Biginelli-like scaffolds. J. Iran. Chem. Soc. 2010, 7, 269–274. [Google Scholar] [CrossRef]
- Wang, Z.-T.; Xu, L.-W.; Xia, C.-G.; Wang, H.-Q. Novel Biginelli-like three-component cyclocondensation reaction: Efficient synthesis of 5-unsubstituted 3,4-dihydropyrimidin-2(1H)-ones. Tetrahedron Lett. 2004, 45, 7951–7953. [Google Scholar] [CrossRef]
- Wang, M.; Song, J.; Lu, Q.; Wang, Q. Green Biginelli-type reaction: Solvent-free synthesis of 5-unsubstituted 3,4-dihydropyrimdin-2(1H)-ones. J. Heterocycl. Chem. 2015, 52, 1907–1910. [Google Scholar] [CrossRef]
- Gore, S.; Baskaran, S.; Koenig, B. Efficient synthesis of 3,4-dihydropyrimidin-2-ones in low melting tartaric acid–urea mixtures. Green. Chem. 2011, 13, 1009–1013. [Google Scholar] [CrossRef]
- Murata, H.; Ishitani, H.; Iwamoto, M. Synthesis of Biginelli dihydropyrimidinone derivatives with various substituents on aluminium-planted mesoporous silica catalyst. Org. Biomol. Chem. 2010, 8, 1202–1211. [Google Scholar] [CrossRef] [PubMed]
- Hu, E.H.; Sidler, D.R.; Dolling, U.H. Unprecedented catalytic three component one-pot condensation reaction: An efficient synthesis of 5-alkoxycarbonyl-4-aryl-3,4-dihydropyrimidin-2(1H)-ones. J. Org. Chem. 1998, 63, 3454–3457. [Google Scholar] [CrossRef]
- Lu, J.; Bai, Y. Catalysis of the Biginelli reaction by ferric and nickel chloride hexahydrates. One-pot synthesis of 3,4-dihydropyrimidin-2(1H)-ones. Synthesis 2002, 12, 466–470. [Google Scholar] [CrossRef]
- Ranu, B.C.; Hajra, A.; Jana, U. Indium(III) chloride-catalyzed one-pot synthesis of dihydropyrimidinones by a three-component coupling of 1,3-dicarbonyl compounds, aldehydes, and urea: An improved procedure for the Biginelli reaction. J. Org. Chem. 2000, 65, 6270–6272. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Wang, Y.-G. A rapid and efficient Biginelli reaction catalyzed by zinc triflate. Chin. J. Chem. 2003, 21, 327–331. [Google Scholar]
- Paraskar, A.S.; Dewkar, G.K.; Sudalai, A. Cu(OTf)2: A reusable catalyst for high-yield synthesis of 3,4-dihydropyrimidin-2(1H)-ones. Tetrahedron Lett. 2003, 44, 3305–3308. [Google Scholar] [CrossRef]
- Dondoni, A.; Massi, A. Parallel synthesis of dihydropyrimidinones using Yb(III)-resin and polymer-supported scavengers under solvent-free conditions. A green chemistry approach to the Biginelli reaction. Tetrahedron Lett. 2001, 42, 7975–7978. [Google Scholar] [CrossRef]
- Chen, R.-F.; Qian, C.-T. One-pot syntheses of 3,4-dihydropyrimidine-2(1H)-thiones catalyzed by La(OTf)3. Chin. J. Chem. 2002, 20, 427–430. [Google Scholar] [CrossRef]
- Sabitha, G.; Reddy, G.S.K.K.; Reddy, C.S.; Yadav, J.S. One-pot synthesis of dihydropyrimidinones using iodotrimethylsilane. Facile and new improved protocol for the Biginelli reaction at room temperature. Synlett 2003, 858–860. [Google Scholar] [CrossRef]
- Kawade, D.S.; Chaudhari, M.A.; Gujar, J.B.; Shingare, M.S. DBU: An efficient catalyst for the synthesis of 5-unsubstituted-3,4-dihydropyrimidin-2(1H)-one derivatives under microwave irradiation. Heterocycl. Lett. 2015, 5, 637–643. [Google Scholar]
- Shen, Z.-L.; Xu, X.-P.; Ji, S.-J. Brønsted base-catalyzed one-pot three-component Biginelli-type reaction: An efficient synthesis of 4,5,6-triaryl-3,4-dihydropyrimidin-2(1H)-one and mechanistic study. J. Org. Chem. 2010, 75, 1162–1167. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.-J.; Deng, Y.-Q. Ionic liquids catalyzed Biginelli reaction under solvent-free conditions. Tetrahedron Lett. 2001, 42, 5917–5919. [Google Scholar] [CrossRef]
- Janardhan, B.; Laxmi, S.V.; Rajitha, B. One-pot synthesis of fused 3,4-dihydropyrimidin-2(1H)-ones and thiones using a novel ionic liquid as an efficient and reusable catalyst: Improved protocol conditions for the Biginelli-like scaffolds. Heterocycl. Commun. 2012, 18, 93–97. [Google Scholar] [CrossRef]
- Gupta, R.; Chaudhary, R.P. Ionic liquid-mediated facile synthesis and antimicrobial study of thiazolo[2,3-b]benzo[h]quinazolines and thiazino[2,3-b]benzo-[h]quinazolines. Phosphorus Sulfur 2012, 187, 735–742. [Google Scholar] [CrossRef]
- Legeay, J.-C.; Eynde, J.J.V.; Bazureau, J.P. Ionic liquid phase technology supported the three component synthesis of Hantzsch 1,4-dihydropyridines and Biginelli 3,4-dihydropyrimidin-2(1H)-ones under microwave dielectric heating. Tetrahedron. 2005, 61, 12386–12397. [Google Scholar] [CrossRef]
- Dutta, M.; Gogoi, J.; Shekarrao, K.; Goswami, J.; Gogoi, S.; Boruah, R.C. Simple ultrasound-assisted synthesis of 3,4-dihydropyrimidin-2(1H)-one and 3,4-dihydropyrimidine-2(1H)-thione-fused steroidal derivatives by a three-component reaction. Synthesis 2012, 2614–2622. [Google Scholar] [CrossRef]
- Heirati, S.Z.D.; Shirini, F.; Shojaei, A.F. PEG-SANM nanocomposite: A new catalytic application towards clean and highly efficient Biginelli-like reaction under solvent-free conditions. RSC Adv. 2016, 6, 67072–67085. [Google Scholar] [CrossRef]
- Azarifar, D.; Abbasi, Y.; Badalkhani, O. Sulfonic acid-functionalized titanomagnetite nanoparticles as recyclable heterogeneous acid catalyst for one-pot solvent-free synthesis of 3,4-dihydropyrimidin-2(1H)-ones/thiones. J. Iran. Chem. Soc. 2016, 13, 2029–2038. [Google Scholar] [CrossRef]
- Safari, J.; Gandomi-Ravandi, S. Fe3O4–CNTs nanocomposites: A novel and excellent catalyst in the synthesis of diarylpyrimidinones using grindstone chemistry. RSC Adv. 2014, 4, 11486–11492. [Google Scholar] [CrossRef]
- Lu, J.; Ma, H. Iron(III)-catalyzed synthesis of dihydropyrimidinones. Improved conditions for the Biginelli reaction. Synlett 2000, 63–64. [Google Scholar]
- Sedova, V.F.; Krivopalov, V.P.; Shkurko, O.P. Synthesis of 5-nitro-3,4-dihydropyrimidin-2(1H)-ones catalyzed by metal salts. Retro-Henry reaction with formation of N,N'-disubstituted ureas. Russ. J. Org. Chem. 2007, 43, 90–95. [Google Scholar] [CrossRef]
- Kappe, C.O. Controlled microwave heating in modern organic synthesis. Angew. Chem. Int. Edit. 2004, 43, 6250–6284. [Google Scholar] [CrossRef] [PubMed]
- Kappe, C.O.; Dallinger, D. The impact of microwave synthesis on drug discovery. Nat. Rev. Drug Discov. 2006, 5, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Caddick, S.; Fitzmaurice, R. Microwave enhanced synthesis. Tetrahedron 2009, 65, 3325–3355. [Google Scholar] [CrossRef]
- Stadler, A.; Kappe, C.O. Microwave-mediated Biginelli reactions revisited. On the nature of rate and yield enhancements. J. Chem. Soc. Perk. Trans 2 2000, 1363–1368. [Google Scholar] [CrossRef]
- Mirza-Aghayan, M.; Bolourtchian, M.; Hosseini, M. Microwave-assisted efficient synthesis of dihydropyrimidines in solvent-free condition. Synth. Commun. 2004, 34, 3335–3341. [Google Scholar] [CrossRef]
- Jetti, S.R.; Upadhyaya, A.; Jain, S. 3,4-Hydropyrimidin-2-(1H)one derivatives: Solid silica-based sulfonic acid catalyzed microwave-assisted synthesis and their biological evaluation as antihypertensive and calcium channel blocking agents. Med. Chem. Res. 2014, 23, 4356–4366. [Google Scholar] [CrossRef]
- Rezaei, R.; Mohammadi, M.K.; Khaledi, A. Microwave-assisted solvent-free one-pot Biginelli synthesis of dihydropyrimidinone compounds on melamine-formaldehyde as a solid support. Asian J. Chem. 2013, 25, 4588–4590. [Google Scholar]
- Bigdeli, M.A.; Jafari, S.; Mahdavinia, G.H.; Hazarkhani, H. Trichloroisocyanuric acid, a new and efficient catalyst for the synthesis of dihydropyrimidinones and dihydropyrimidinethiones. Catal. Commun. 2007, 8, 1641–1644. [Google Scholar] [CrossRef]
- Liang, B.; Wang, X.; Wang, J.-X.; Du, Z. New three-component cyclocondensation reaction: Microwave-assisted one-pot synthesis of 5-unsubstituted-3,4-dihydropyrimidin-2(1H)-ones under solvent-free conditions. Tetrahedron 2007, 63, 1981–1986. [Google Scholar] [CrossRef]
- Desai, B.; Dallinger, D.; Kappe, C.O. Microwave-assisted solution phase synthesis of dihydropyrimidine C5 amides and esters. Tetrahedron 2006, 62, 4651–4664. [Google Scholar] [CrossRef]
- Shanmugam, P.; Annie, G.; Perumal, P.T. Synthesis of novel 3,4-dihydropyrimidinones on water soluble solid support catalyzed by indium triflate. J. Heterocycl. Chem. 2003, 40, 879–883. [Google Scholar] [CrossRef]
- Stadler, A.; Kappe, C.O. Automated library generation using sequential microwave-assisted chemistry. Application toward the Biginelli multicomponent condensation. J. Comb. Chem. 2001, 3, 624–630. [Google Scholar] [CrossRef] [PubMed]
- Zhan, H.W.; Wang, J.X.; Wang, X.T. Solvent- and catalyst-free synthesis of dihydropyrimidinethiones in one-pot under focused microwave irradiation conditions. Chin. Chem. Lett. 2008, 19, 1183–1185. [Google Scholar] [CrossRef]
- Zhu, Y.; Pan, Y.; Huang, S. Trimethylsilyl chloride: A facile and efficient reagent for one-pot synthesis of 3,4-dihydropyrimidin-2(1H)-ones. Synth. Commun. 2004, 34, 3167–3174. [Google Scholar] [CrossRef]
- Kefayati, H.; Fakhriyannejad, M.; Mohammadi, A.A. An efficient synthesis of new 3,4-dihydropyrimidin-2(1H)-ones incorporating a phenyl moiety at C-5 and C-6 catalyzed by TMSCl and Co(OAc)2∙4H2O. Phosphorus Sulfur 2009, 184, 1796–1804. [Google Scholar] [CrossRef]
- Heravi, M.M.; Derikvand, F.; Ranjbar, L.; Bamoharram, F.F. H6P2W18O62∙18H2O, a green and reusable catalyst for the three-component, one-pot synthesis of 4,6-diarylpyrimidin-2(1H)-ones under solvent-free conditions. Synth. Commun. 2010, 40, 1256–1263. [Google Scholar] [CrossRef]
- An, L.; Zhang, L.; Zheng, Y.; Xue, Y.; Mou, J.; Liu, L.; Liu, Y. Microwave irradiation assisted selective synthesis of 4,6-diaryl-3,4-dihydropyrimidin-2(1H)-ones and pyrimidin-2(1H)-ones. Chin. J. Org. Chem. 2012, 32, 1108–1111. [Google Scholar] [CrossRef]
- Kefayati, H.; Asghari, F.; Khanjanian, R. 1-Methylimidazolium hydrogen sulfate/chlorotrimethylsilane, an effective catalytic system for the synthesis of 3,4-dihydropyrimidin-2(1H)-ones and hydroquinazoline-2,5-diones. J. Mol. Liq. 2012, 172, 147–151. [Google Scholar] [CrossRef]
- Zavyalov, S.I.; Kulikova, L.B. Trimethylchlorosilane-dimethylformamide, a new system for the Biginelli reaction. Khim. Farm. Zh. 1992, 26, 116–117. [Google Scholar]
- Zhu, Y.; Pan, Y.; Huang, S. Chemoselective multicomponent condensation of 1,3-cyclohexanedione, urea or thiourea with aldehydes: One-pot synthesis of two families of fused heterocyclic and spiro-fused heterocyclic aliphatic rings. Heterocycles 2005, 65, 133–142. [Google Scholar] [CrossRef]
- Kantevari, S.; Bantu, R.; Nagarapu, L. TMSCl mediated highly efficient one-pot synthesis of octahydroquinazolinone and 1,8-dioxo-octahydroxanthene derivatives. Arkivoc 2006, 16, 136–148. [Google Scholar]
- Ryabukhin, S.V.; Plaskon, A.S.; Ostapchuk, E.N.; Volochnyuk, D.M.; Tolmachev, A.A. N-Substituted ureas and thioureas in Biginelli reaction promoted by chlorotrimethylsilane: Convenient synthesis of N1-alkyl-, N1-aryl-, and N1,N3-dialkyl-3,4-dihydropyrimidin-2(1H)-(thi)ones. Synthesis 2007, 417–427. [Google Scholar] [CrossRef]
- Prokopcová, H.; Pisani, L.; Kappe, C.O. Synthesis of 5-aroyldihydropyrimidinones via Liebeskind-Srogl thiol ester-boronic acid cross-couplings. Synlett 2006, 43–46. [Google Scholar] [CrossRef]
- Matloobi, M.; Kappe, C.O. Microwave-assisted solution- and solid-phase synthesis of 2-amino-4-arylpyrimidine derivatives. J. Comb. Chem. 2007, 9, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Kremsner, J.M.; Stadler, A.; Kappe, C.O. High-throughput microwave assisted organic synthesis: Moving from automated sequential to parallel library-generation formats in silicon carbide microtiter plates. J. Comb. Chem. 2007, 9, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Pisani, L.; Prokopcová, H.; Kremsner, J.M.; Kappe, C.O. 5-Aroyl-3,4-dihydropyrim idin-2-one library generation via automated sequential and parallel microwave-assisted synthesis techniques. J. Comb. Chem. 2007, 9, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Bose, D.S.; Kumar, R.K.; Fatima, L. A remarkable rate acceleration of the one-pot three-component cyclocondensation reaction at room temperature: An expedient synthesis of mitotic kinesin Eg5 inhibitor monastrol. Synlett 2004, 279–282. [Google Scholar] [CrossRef]
- Chebanov, V.A.; Saraev, V.E.; Desenko, S.M.; Chernenko, V.N.; Knyazeva, I.V.; Groth, U.; Glasnov, T.N.; Kappe, C.O. Tuning of chemoand regioselectivities in multicomponent condensations of 5-aminopyrazoles, dimedone, and aldehydes. J. Org. Chem. 2008, 73, 5110–5118. [Google Scholar] [CrossRef] [PubMed]
- Ryabukhin, S.V.; Plaskon, A.S.; Ostapchuk, E.N.; Volochnyuk, D.M.; Shishkin, O.V.; Tolmachev, A.A. CF3-substituted 1,3-dicarbonyl compounds in the Biginelli reaction promoted by chlorotrimethylsilane. J. Fluorine Chem. 2008, 129, 625–631. [Google Scholar] [CrossRef]
- Nagarapu, L.; Bantu, R.; Mereyala, H.B. TMSCl-mediated one-pot, threecomponent synthesis of 2H-Indazolo[2,1-b]phthalazine-triones. J. Heterocyl. Chem. 2009, 46, 728–731. [Google Scholar] [CrossRef]
- Azizian, J.; Mirza, B.; Mohtahedi, M.M.; Abaee, M.S.; Sargordan, M. Biginelli reaction for synthesis of novel trifluoromethyl derivatives of bis(tetrahydropyrimidinone)benzenes. J. Fluorine Chem. 2008, 129, 1083–1089. [Google Scholar] [CrossRef]
- Sabitha, G.; Reddy, G.S.K.K.; Reddy, C.S.; Yadav, J.S. A novel TMSI-mediated synthesis of Hantzsch 1,4-dihydropyridines at ambient temperature. Tetrahedron Lett. 2003, 44, 4129–4131. [Google Scholar] [CrossRef]
- Sabitha, G.; Reddy, K.B.; Srinivas, R.; Yadav, J.S. Iodotrimethylsilane-accelerated one-pot synthesis of 5-unsubstituted 3,4-dihydropyrimidin-2(1H)-ones: A novel procedure for the Biginelli-like cyclocondensation reaction at room temperature. Helv. Chim. Acta 2005, 88, 2996–2999. [Google Scholar] [CrossRef]
- Wan, J.-P.; Liu, Y. Multicomponent reactions promoted by organosilicon reagents. Curr. Org. Chem. 2011, 15, 2758–2773. [Google Scholar] [CrossRef]
- Bandini, M.; Fagioli, M.; Melloni, A.; Umani-Ronchi, A. A general procedure for the synthesis of 1,3-bis(indolyl) compounds via Michael addition catalyzed by InBr3/TMSCl. Synthesis 2003, 397–402. [Google Scholar] [CrossRef]
- Ito, T.; Ishino, Y.; Mizuno, T.; Iswkawa, A.; Kobayashi, J. Zinc metal-promoted cross-coupling reaction of non-activated alkyl halides with aldehydes in the presence of chlorotrimethylsilane. Synlett 2002, 2116–2118. [Google Scholar] [CrossRef]
- Lee, P.H.; Ahn, H.; Lee, K.; Sung, S.; Kim, S. Studies on the reactions of α,β-enones with allyl indium reagent: Effects of TMSCl as promoter on regioselectivity. Tetrahedron Lett. 2001, 42, 37–39. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds 4 and 6 are available from the authors. |

| Entry | Catalyst | Additive | Solvent | Time | Yield (%) b |
|---|---|---|---|---|---|
| 1 c | FeCl3∙6H2O | - | CH3CN | 10 h | 38 |
| 2 | FeCl3∙6H2O | - | CH3CN | 2 h | 37 |
| 3 | ZnCl2 | - | CH3CN | 2 h | trace |
| 4 | FeSO4∙7H2O | - | CH3CN | 2 h | trace |
| 5 | CuBr2 | - | CH3CN | 2 h | 16 |
| 6 | AlCl3 | - | CH3CN | 2 h | 18 |
| 7 | FeCl3∙6H2O | BF3∙OEt2 | CH3CN | 2 h | 43 |
| 8 | FeCl3∙6H2O | BBr3 | CH3CN | 2 h | 41 |
| 9 | FeCl3∙6H2O | TMSOTf | CH3CN | 2 h | 65 |
| 10 | FeCl3∙6H2O | TMSCl | CH3CN | 2 h | 82 |
| 11 | FeCl3∙6H2O | TMSBr | CH3CN | 2 h | 88 |
| 12 | FeCl3∙6H2O | TMSI | CH3CN | 2 h | 73 |
| 13 | FeCl3∙6H2O | TMSBr | Toluene | 2 h | 44 |
| 14 | FeCl3∙6H2O | TMSBr | THF | 2 h | 61 |
| 15 | FeCl3∙6H2O | TMSBr | 1,4-Dioxane | 2 h | 71 |
| 16 | FeCl3∙6H2O | TMSBr | EtOH | 2 h | 84 |
| 17 | FeCl3∙6H2O | TMSBr | neat | 2 h | 52 |
| 18 c | FeCl3∙6H2O | TMSBr | CH3CN | 8 h | 80 |
| 19 c | FeCl3∙6H2O | TMSBr | CH3CN | 10 h | 87 |

| Entry | Ketones (1) | Benzaldehydes (2) | Products (4) | Yield (%) b |
|---|---|---|---|---|
| 1 |  |  |  | 88 |
| 2 |  |  |  | 86 |
| 3 |  |  |  | 83 |
| 4 |  |  |  | 80 |
| 5 |  |  |  | 91 |
| 6 |  |  |  | 76 |
| 7 |  |  |  | 89 |
| 8 |  |  |  | 90 |
| 9 |  |  |  | 90 |
| 10 |  |  |  | 84 |
| 11 |  |  |  | 86 |
| 12 |  |  |  | 92 |
| 13 |  |  |  | 89 |

| Entry | Ketones (1) | Benzaldehydes (2) | Products (6) | Yield (%) b |
|---|---|---|---|---|
| 1 |  |  |  | 90 |
| 2 |  |  |  | 86 |
| 3 |  |  |  | 91 |
| 4 |  |  |  | 84 |
| 5 |  |  |  | 82 |
| 6 |  |  |  | 90 |
| 7 |  |  |  | 80 |
| 8 |  |  |  | 81 |
| 9 |  |  |  | 83 |
| 10 |  |  |  | 88 |
| 11 |  |  |  | 87 |
| 12 |  |  |  | 82 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, F.; Jia, X.; Li, P.; Zhao, J.; Huang, J.; Li, H.; Li, L. FeCl3∙6H2O/TMSBr-Catalyzed Rapid Synthesis of Dihydropyrimidinones and Dihydropyrimidinethiones under Microwave Irradiation. Molecules 2017, 22, 1503. https://doi.org/10.3390/molecules22091503
Zhao F, Jia X, Li P, Zhao J, Huang J, Li H, Li L. FeCl3∙6H2O/TMSBr-Catalyzed Rapid Synthesis of Dihydropyrimidinones and Dihydropyrimidinethiones under Microwave Irradiation. Molecules. 2017; 22(9):1503. https://doi.org/10.3390/molecules22091503
Chicago/Turabian StyleZhao, Fei, Xiuwen Jia, Pinyi Li, Jingwei Zhao, Jun Huang, Honglian Li, and Lin Li. 2017. "FeCl3∙6H2O/TMSBr-Catalyzed Rapid Synthesis of Dihydropyrimidinones and Dihydropyrimidinethiones under Microwave Irradiation" Molecules 22, no. 9: 1503. https://doi.org/10.3390/molecules22091503