The Antioxidant Activity and Their Major Antioxidant Compounds from Acanthopanax senticosus and A. koreanum
Abstract
:1. Introduction
2. Results and Discussion
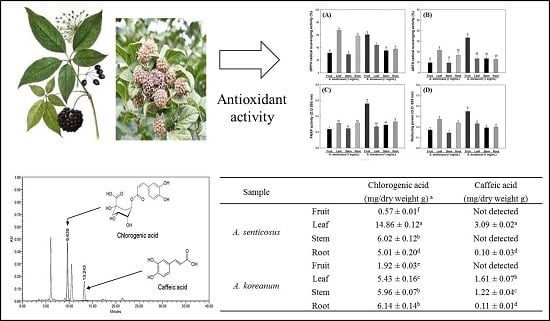
2.1. Antioxidant Activity from Various Parts of A. Senticosus and A. Koreanum Extracts

2.2. Total Phenolic, Flavonoid and Proanthocyanidin Contents
| Sample | Total Phenolic Contents (mg GAE/dry weight g) | Total Flavonoid Contents (mg RE/dry weight g) | Proanthocyanidin (mg CE/dry weight g) | |
|---|---|---|---|---|
| A. senticosus | Fruit | 25.70 ± 0.69 f | 16.44 ± 0.99 g | 10.86 ± 0.49 c |
| Leaf | 56.08 ± 0.47 b | 41.23 ± 1.98 a | 8.75 ± 1.02 d | |
| Stem | 23.50 ± 0.42 g | 11.49 ± 1.49 h | 2.88 ± 0.28 f | |
| Root | 44.00 ± 0.14 c | 36.49 ± 0.37 b,c | 7.61 ± 0.56 e | |
| A. koreanum | Fruit | 73.34 ± 1.16 a | 31.31 ± 0.37 d | 33.01 ± 1.02 a |
| Leaf | 40.61 ± 1.03 d | 38.21 ± 0.65 b | 6.79 ± 0.28 e | |
| Stem | 35.45 ± 0.47 e | 21.18 ± 0.99 f | 7.44 ± 0.98 e | |
| Root | 34.40 ± 0.69 e | 23.56 ± 1.63 e | 3.86 ± 0.28 f | |
2.3. Relationship between Antioxidant Activity and Antioxidant Compounds
| Parameter | TPC | TFC | PC | DPPH | ABTS | FRAP | Reducing Power | ORAC |
|---|---|---|---|---|---|---|---|---|
| TPC | 1 | 0.688 | 0.802 * | 0.867 ** | 0.981 ** | 0.876 ** | 0.992 ** | 0.922 ** |
| TFC | 0.688 | 1 | 0.218 | 0.866 ** | 0.539 | 0.321 | 0.717 * | 0.637 |
| PC | 0.802 * | 0.218 | 1 | 0.480 | 0.877 ** | 0.896 ** | 0.812 * | 0.769 * |
| DPPH | 0.867 ** | 0.866 ** | 0.480 | 1 | 0.792 * | 0.580 | 0.861 ** | 0.759 * |
| ABTS | 0.981 ** | 0.539 | 0.877 ** | 0.792 * | 1 | 0.936 ** | 0.966 ** | 0.911 ** |
| FRAP | 0.876 ** | 0.321 | 0.896 ** | 0.580 | 0.936 ** | 1 | 0.869 ** | 0.883 ** |
| Reducing power | 0.992 ** | 0.717 * | 0.812 * | 0.861 ** | 0.966 ** | 0.869 ** | 1 | 0.927 ** |
| ORAC | 0.922 ** | 0.637 | 0.769 * | 0.759 * | 0.911 ** | 0.883 ** | 0.927 ** | 1 |
2.4. The Chlorogenic Acid and Caffeic Acid Contents

| Sample | Chlorogenic Acid (mg/dry weight g) | Caffeic Acid (mg/dry weight g) | |
|---|---|---|---|
| A. senticosus | Fruit | 0.57 ± 0.01 f | Not detected |
| Leaf | 14.86 ± 0.12 a | 3.09 ± 0.02 a | |
| Stem | 6.02 ± 0.12 b | Not detected | |
| Root | 5.01 ± 0.20 d | 0.10 ± 0.03 d | |
| A. koreanum | Fruit | 1.92 ± 0.03 e | Not detected |
| Leaf | 5.43 ± 0.16 c | 1.61 ± 0.07 b | |
| Stem | 5.96 ± 0.07 b | 1.22 ± 0.04 c | |
| Root | 6.14 ± 0.14 b | 0.11 ± 0.01 d | |
2.5. Method Validation
2.5.1. Specificity
2.5.2. Evaluation of Accuracy and Precision, Limit of Detection, and Limit of Quantification
| Parameters | Chlorogenic Acid (mg/dry weight g) | Caffeic Acid (mg/dry weight g) | ||
|---|---|---|---|---|
| Mean ± SD | RSD (%) | Mean ± SD | RSD (%) | |
| Inter-day | 15.15 ± 0.13 | 0.87 | 3.32 ± 0.05 | 1.39 |
| Intra-day | 15.56 ± 0.26 | 1.70 | 3.43 ± 0.05 | 1.47 |
| Added (mg/dry weight g) | Recovery (%) | Values of LOD and LOQ (μg/mL) | |||
|---|---|---|---|---|---|
| Mean ± SD | RSD (%) | LOD | LOQ | ||
| Chlorogenic acid | 15.15 (100%) | 110.74 ± 2.84 | 2.56 | - | - |
| 7.58 (50%) | 105.01 ± 0.97 | 0.92 | - | - | |
| 3.79 (25%) | 106.00 ± 3.29 | 3.10 | 0.1 | 0.3 | |
| Caffeic acid | 3.32 (100%) | 96.41 ± 1.84 | 1.91 | - | - |
| 1.66 (50%) | 92.49 ± 0.75 | 0.81 | - | - | |
| 0.83 (25%) | 91.29 ± 0.47 | 0.52 | 0.03 | 0.06 | |
3. Experimental Section
3.1. Sample Preparation
3.2. Chemicals
3.3. Antioxidant Activity
3.3.1. DPPH Radical Scavenging Activity
3.3.2. ABTS Radical Scavenging Activity
3.3.3. Ferric Reducing Antioxidant Power (FRAP)
3.3.4. Reducing Power
3.3.5. Oxygen Radical Absorbance Capacity (ORAC)
3.3.6. Total Phenolic Content (TPC)
3.3.7. Total Flavonoid Content (TFC)
3.3.8. Proanthocyanidin Content (PC)
3.4. HPLC Analysis of Phenolic Compounds
3.5. Validation Procedure
3.5.1. Specificity
3.5.2. Linearity
3.5.3. Precision and Accuracy
3.5.4. Limit of Detection (LOD) and Limit of Quantification (LOQ)
3.6. Statistical Analysis
4. Conclusions
Acknowledgement
Author Contributions
Conflicts of Interest
References
- Chen, C.Y.O.; Ribaya-Mercado, J.D.; McKay, D.L.; Croom, E.; Blumberg, J.B. Differential antioxidant and quinone reductase inducing activity of American, Asian, and Siberian ginseng. Food Chem. 2010, 119, 445–451. [Google Scholar] [CrossRef]
- Yu, C.Y.; Kim, S.H.; Lim, J.D.; Kim, M.J.; Chung, I.M. Intraspecific relationship analysis by DNA markers and in vitro cytotoxic and antioxidant activity in Eleutherococcus senticosus. Toxicol. in Vitro 2003, 17, 229–236. [Google Scholar] [CrossRef]
- Lin, Q.Y.; Jin, L.J.; Cao, Z.H.; Li, H.Q.; Xu, Y.P. Protective effect of Acanthopanax senticosus extract against endotoxic shock in mice. J. Ethnopharmacol. 2008, 118, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.; Um, M.Y.; Lee, H.; Jung, C.H.; Heo, S.H.; Ha, T.Y. Eleutheroside E, an active component of Eleutherococcus senticosus, ameliorates insulin resistance in type 2 diabetic db/db mice. Evid. Based Complement. Altern. Med. 2013, 2013, 934183. [Google Scholar] [CrossRef] [PubMed]
- Rates, S.M.K. Plants as source of drugs. Toxicon 2001, 39, 603–613. [Google Scholar] [CrossRef]
- Huang, L.; Zhao, H.; Huang, B.; Zheng, C.; Peng, W.; Qin, L. Acanthopanax senticosus: Review of botany, chemistry and pharmacology. Pharmazie 2011, 66, 83–97. [Google Scholar] [PubMed]
- Kang, J.S.; Linh, P.T.; Cai, X.F.; Kim, H.S.; Lee, J.J.; Kim, Y.H. Quantitative determination of eleutheroside B and E from Acanthopanax species by high performance liquid chromatography. Arch. Pharm. Res. 2001, 24, 407–411. [Google Scholar] [CrossRef] [PubMed]
- Olthof, M.R.; Hollman, P.C.H.; Katan, M.B. Chlorogenic acid and caffeic acid are absorbed in humans. J. Nutr. 2001, 131, 66–71. [Google Scholar] [PubMed]
- Sato, Y.; Itagaki, S.; Kurokawa, T.; Ogura, J.; Kobayashi, M.; Hirano, T.; Sugawara, M.; Iseki, K. In vitro and in vivo antioxidant properties of chlorogenic acid and caffeic acid. Int. J. Pharm. 2011, 403, 136–138. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Bae, D.B.; Lee, J.S.; Park, S.O.; Lee, S.J.; Cho, O.H.; Lee, O.H. Determination of eleutherosides and β-glucan content from different parts and cultivating areas of A senticosus and A. koreanum. J. Korean Soc. Food Sci. Nutr. 2013, 42, 2082–2087. [Google Scholar] [CrossRef]
- Ong, E.S. Extraction methods and chemical standardization of botanicals and herbal preparations. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2004, 812, 23–33. [Google Scholar] [CrossRef]
- Li, Q.; Jia, Y.; Xu, L.; Wang, X.; Shen, Z.; Liu, Y.; Bi, K. Simultaneous determination of protocatechuic acid, syringin, chlorogenic acid, caffeic acid, liriodendrin and isofraxidin in Acanthopanax senticosus Harms by HPLC-DAD. Biol. Pharm. Bull. 2006, 29, 532–534. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Zheng, C.; Huang, J.; You, T. Identification of herb Acanthopanax senticosus (Rupr. Et Maxim.) harms by capillary electrophoresis with electrochemical detection. Anal. Sci. 2007, 23, 705–711. [Google Scholar] [CrossRef]
- Finkel, T.; Holbrook, N.J. Oxidants, oxidative stress and the biology of ageing. Nature 2000, 408, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Kim, D.B.; Lee, J.S.; Cho, J.H.; Kim, B.K.; Choi, H.S.; Lee, B.Y.; Lee, O.H. Antioxidant activity and anti-adipogenic effects of wild herbs mainly cultivated in Korea. Molecules 2013, 18, 12937–12950. [Google Scholar] [CrossRef] [PubMed]
- Moo-Huchin, V.M.; Moo-Huchin, M.I.; Estrada-Leon, R.J.; Cuevas-Glory, L.; Estrada-Mota, I.A.; Ortiz-Vazquez, E.; Betancur-Ancona, D.; Sauri-Duch, E. Antioxidant compounds, antioxidant activity and phenolic content in peel from three tropical fruits from Yucatan, Mexico. Food Chem. 2015, 166, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Hanasaki, Y.; Ogawa, S.; Fukui, S. The correlation between active oxygens scavenging and antioxidative effects of flavonoids. Free Radic. Biol. Med. 1994, 16, 845–850. [Google Scholar] [CrossRef]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef] [PubMed]
- Cho, M.L.; Lee, H.S.; Kang, I.J.; Won, M.H.; You, S.G. Antioxidant properties of extract and fractions from Enteromorpha prolifera, a type of green seaweed. Food Chem. 2011, 127, 999–1006. [Google Scholar] [CrossRef] [PubMed]
- Heo, S.J.; Ahn, H.Y.; Kang, M.J.; Lee, J.H.; Cha, J.Y.; Cho, Y.S. Antioxidative activity and chemical characteristics of leaves, roots, stems and fruits extracts from Acanthopanax senticosus. J. Life Sci. 2011, 21, 1052–1059. [Google Scholar] [CrossRef]
- Fernando, I.; Abeysinghe, D.; Dharmadasa, R. Determination of phenolic contents and antioxidant capacity of different parts of Withania somnifera (L.) Dunal. from three different growth stages. Ind. Crops Prod. 2013, 50, 537–539. [Google Scholar]
- Lachman, J. Major factors influencing antioxidant contents and antioxidant activity in grapes and wines. Int. J. Wine Res. 2009, 1, 101–121. [Google Scholar] [CrossRef]
- Adedapo, A.A.; Jimoh, F.O.; Afolayan, A.J.; Masika, P.J. Antioxidant properties of the methanol extracts of the leaves and stems of Celtis africana. Rec. Nat. Prod. 2009, 3, 23–31. [Google Scholar]
- Dudonne, S.; Vitrac, X.; Coutiere, P.; Woillez, M.; Merillon, J.M. Comparative study of antioxidant properties and total phenolic content of 30 plant extracts of industrial interest using DPPH, ABTS, FRAP, SOD, and ORAC assays. J. Agric. Food Chem. 2009, 57, 1768–1774. [Google Scholar] [CrossRef] [PubMed]
- Skerget, M.; Kotnik, P.; Hadolin, M.; Hras, A.R.; Simonic, M.; Knez, Z. Phenols, proanthocyanidins, flavones and flavonols in some plant materials and their antioxidant activities. Food Chem. 2005, 89, 191–198. [Google Scholar] [CrossRef]
- Dumas, Y.; Dadomo, M.; Lucca, G.D.; Grolier, P. Effects of environmental factors and agricultural techniques on antioxidant content of tomatoes. J. Sci. Food Agric. 2003, 83, 369–382. [Google Scholar] [CrossRef]
- KFDA (Korea Food and Drug Administration). Analytical Method Guideline about Validation of Drugs and etc.; Korea Food & Drug Administration: Osong, Korea, 2012; pp. 1–26. [Google Scholar]
- Gadow, A.; Joubert, E.; Hansmann, C.F. Comparison of the antioxidant activity of aspalathin with that of other plant phenols of rooibos tea (Aspalathus linearis), α-tocopherol, BHT, and BHA. J. Agric. Food Chem. 1997, 45, 632–638. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, I.C.; Baptista, P.; Vilas-Boas, M.; Barros, L. Free-radical scavenging capacity and reducing power of wild edible mushrooms from northeast Portugal: Individual cap and stipe activity. Food Chem. 2007, 100, 1511–1516. [Google Scholar] [CrossRef]
- Ou, B.; Hampsch-Woodill, M.; Prior, R.L. Development and validation of an improved oxygen radical absorbance capacity assay using fluorescein as the fluorescent probe. J. Agric. Food Chem. 2001, 49, 4619–4626. [Google Scholar] [CrossRef] [PubMed]
- Tezcan, F.; Gultekin-Ozguven, M.; Diken, T.; Ozcelik, B.; Erim, F.B. Antioxidant activity and total phenolic, organic acid and sugar content in commercial pomegranate juices. Food Chem. 2009, 115, 873–877. [Google Scholar] [CrossRef]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Mitsunaga, T.; Doi, T.; Kondo, Y.; Abe, I. Color development of proanthocyanidins in vanillin-hydrochloric acid reaction. J. Wood Sci. 1998, 44, 125–130. [Google Scholar] [CrossRef]
- Sample Availability: Samples of different parts extracts from Acanthopanax senticosus and A. senticosus are available from authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.-H.; Cho, M.L.; Kim, D.-B.; Shin, G.-H.; Lee, J.-H.; Lee, J.S.; Park, S.-O.; Lee, S.-J.; Shin, H.M.; Lee, O.-H. The Antioxidant Activity and Their Major Antioxidant Compounds from Acanthopanax senticosus and A. koreanum. Molecules 2015, 20, 13281-13295. https://doi.org/10.3390/molecules200713281
Kim Y-H, Cho ML, Kim D-B, Shin G-H, Lee J-H, Lee JS, Park S-O, Lee S-J, Shin HM, Lee O-H. The Antioxidant Activity and Their Major Antioxidant Compounds from Acanthopanax senticosus and A. koreanum. Molecules. 2015; 20(7):13281-13295. https://doi.org/10.3390/molecules200713281
Chicago/Turabian StyleKim, Young-Hyun, Myoung Lae Cho, Dan-Bi Kim, Gi-Hae Shin, Jin-Ha Lee, Jong Seok Lee, Sun-Ok Park, Sang-Jong Lee, Hyun Mu Shin, and Ok-Hwan Lee. 2015. "The Antioxidant Activity and Their Major Antioxidant Compounds from Acanthopanax senticosus and A. koreanum" Molecules 20, no. 7: 13281-13295. https://doi.org/10.3390/molecules200713281