Novel Polyprenylated Phloroglucinols from Hypericum sampsonii
Abstract
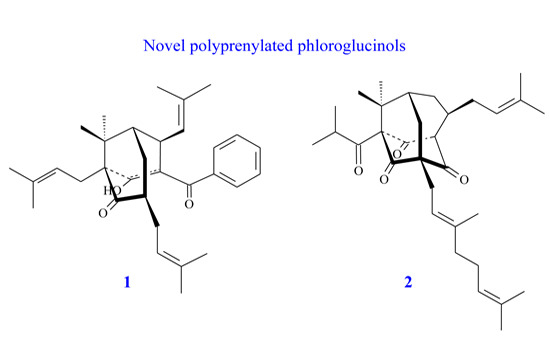
:1. Introduction


2. Results and Discussion

| Position | δH | |||
|---|---|---|---|---|
| 1 a | 2 a | |||
| 1H | 13C | 1H | 13C | |
| 1 | 209.4 s | 202.2 s | ||
| 2 | 65.4 s | 88.8 s | ||
| 3 | 185.5 s | 205.4 s | ||
| 4 | 111.4 s | 2.97 m | 50.0 d | |
| 5 | 204.7 s | |||
| 6 | 2.50 m | 45.5 d | 69.2 s | |
| 7 | 1.44 m | 29.9 t | 1.87 d (10.5) | 36.2 t |
| 2.06 m | 2.50 m | |||
| 8 | 1.50 m | 45.3 d | 1.75 m | 41.4 d |
| 9 | 42.4 s | 50.0 s | ||
| 10 | 1.26 s | 26.0 q | 1.20 s | 24.8 q |
| 11 | 0.92 s | 25.1 q | 1.18 s | 24.2 q |
| 12 | 4.00 dd (7.8, 7.0) | 35.4 d | 1.84 d (7.8) | 35.4 d |
| 2.74 m | ||||
| 13 | 4.63 d (7.8) | 125.7 d | 2.81 m | 58.1 d |
| 14 | 132.7 s | 2.06 m | 31.1 t | |
| 2.30 m | ||||
| 15 | 1.24 s | 25.1 q | 4.97 t (6.5) | 119.9 d |
| 16 | 1.18 s | 17.3 q | 135.8 s | |
| 17 | 195.2 s | 1.67 s | 25.8 q | |
| 18 | 139.3 s | 1.56 s | 17.9 q | |
| 19 | 7.36 m | 128.0 d | 2.50 m | 32.3 t |
| 2.66 dd (11.5, 7.0) | ||||
| 20 | 7.39 m | 126.7 d | 4.99 t (7.0) | 119.3 d |
| 21 | 7.36 m | 130.1 d | 139.6 s | |
| 22 | 7.39 m | 126.7 d | 1.61 s | 16.4 q |
| 23 | 7.36 m | 128.0 d | 2.01 m | 40.1 t |
| 24 | 1.88 m | 28.3 t | 2.03 m | 26.6 t |
| 2.32 m | ||||
| 25 | 4.98 t (6.5) | 121.3 d | 5.00 t (6.5) | 124.1 d |
| 26 | 133.2 s | 131.5 s | ||
| 27 | 1.65 s | 26.0 q | 1.64 s | 25.7 q |
| 28 | 1.54 s | 17.9 q | 1.56 s | 17.6 q |
| 29 | 2.57 dd (13.0, 7.0) | 26.3 t | 211.5 s | |
| 2.89 br d (13.0) | ||||
| 30 | 5.15 br s | 122.9 d | 2.45 hepta (7.0) | 42.8 d |
| 31 | 130.3 s | 1.19 d (7.0) | 21.0 q | |
| 32 | 1.66 s | 25.7 q | 1.21 d (7.0) | 21.0 q |
| 33 | 1.56 s | 17.9 q | ||
| OH | 16.25 s | |||


3. Experimental Section
3.1. General Experimental Procedures
3.2. Plant Material
3.3. Extraction and Isolation
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chiu, N.Y.; Chang, K.H. The Illustrated Medicinal Plants of Taiwan; SMC Pubishing Inc.: Taipei, Taiwan, 1986; Volume II, p. 126. [Google Scholar]
- Chen, M.T.; Chen, C.M. Xanthones from Hypericum sompsonii. Heterocycles 1985, 23, 2543–2548. [Google Scholar] [CrossRef]
- Hu, L.H.; Yip, S.C.; Sim, K.Y. Xanthones from Hypericum ascyron. Phytochemistry 1999, 52, 1371–1373. [Google Scholar] [CrossRef]
- Hong, D.; Yin, F.; Hu, L.H.; Lu, P. Sulfonated xanthones from Hypericum sampsonii. Phytochemistry 2004, 65, 2595–2598. [Google Scholar] [CrossRef] [PubMed]
- Don, M.J.; Huang, Y.J.; Huang, R.L.; Lin, Y.L. New Phenolic principles from Hypericum sampsonii. Chem. Pharm. Bull. 2004, 52, 866–869. [Google Scholar] [CrossRef] [PubMed]
- Kitanov, G.M.; Nedialkov, P.T. Benzophenone O-glucoside, a biogenic precursor of 1,3,7-trioxygenated xanthones in Hypericum annulatum. Phytochemistry 2001, 57, 1237–1243. [Google Scholar] [CrossRef] [PubMed]
- Wirz, A.; Simmen, U.; Heilmann, J.; Calis, I.; Meier, B.; Sticher, O. Bisanthraquinone glycosides of Hypericum perforatum with binding inhibition to CRH-1 receptors. Phytochemistry 2000, 55, 941–947. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.H.; Sim, K.Y. Prenylated phloroglucinol derivatives from Hypericum sampsonii. Tetrahedron Lett. 1998, 39, 7999–8002. [Google Scholar] [CrossRef]
- Hu, L.H.; Sim, K.Y. Complex caged polyisoprenylated benzophenone derivatives, sampsoniones A and B, from Hypericum sampsonii. Tetrahedron Lett. 1999, 40, 759–762. [Google Scholar] [CrossRef]
- Hu, L.H.; Sim, K.Y. Cytotoxic polyprenylated benzoylphloroglucinol derivatives with an unusual adamantly skeleton from Hypericum sampsonii (Guttiferae). Org. Lett. 1999, 1, 879–882. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.H.; Sim, K.Y. Sampsoniones A–M, a unique family of caged polyprenylated benzoylphloroglucinol derivatives, from Hypericum sampsonii. Tetrahedron 2000, 56, 1379–1386. [Google Scholar] [CrossRef]
- Shan, M.D.; Hu, L.H.; Chen, Z.L. Three new hyperforin analogues from Hypericum perforatum. J. Nat. Prod. 2001, 64, 127–130. [Google Scholar] [CrossRef] [PubMed]
- Matsuhisa, M.; Shikishima, Y.; Takaishi, Y.; Honda, G.; Ito, M.; Takeda, Y.; Shibata, H.; Higuti, T.; Kodzhimatov, O.K.; Ashurmrtov, O. Benzoylphloroglucinol derivatives from Hypericum scabrum. J. Nat. Prod. 2002, 65, 290–294. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.L.; Wu, Y.S. Polyprenylated phloroglucinol derivatives from Hypericum sampsonii. Helv. Chim. Acta 2003, 86, 2156–2163. [Google Scholar] [CrossRef]
- Xiao, Z.Y.; Mu, Q.; Shiu, W.K.P.; Zeng, Y.H.; Gibbons, S. Polyisoprenylated benzoyl-phloroglucinol derivatives from Hypericum sampsonii. J. Nat. Prod. 2007, 70, 1779–1782. [Google Scholar] [CrossRef] [PubMed]
- Xin, W.B.; Man, X.H.; Zheng, C.J.; Jia, M.; Jiang, Y.P.; Zhao, X.X.; Jin, G.L.; Mao, Z.J.; Huang, H.Q.; Qin, L.P. Prenylated phloroglucinol derivatives from Hypericum sampsonii. Fitoterapia 2012, 83, 1540–1547. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, N.; Takaishi, Y.; Shikishima, Y.; Nakanishi, Y.; Bastow, K.; Lee, K.H.; Honda, G.; Ito, M.; Takeda, Y.; Kodzhimatov, O.K.; et al. Prenylated benzophenones and xanthones from Hypericum scabrum. J. Nat. Prod. 2004, 67, 1870–1875. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.H.; Osman, K.; Xiao, Z.Y.; Gibbons, S.; Mua, Q. Four geranyl-bearing polyisopre-nylated benzoylphloroglucinol derivatives from Hypericum sampsonii. Phytochem. Lett. 2012, 5, 200–205. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the all compounds are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.-J.; Chen, H.-J.; Lin, Y.-L. Novel Polyprenylated Phloroglucinols from Hypericum sampsonii. Molecules 2014, 19, 19836-19844. https://doi.org/10.3390/molecules191219836
Chen J-J, Chen H-J, Lin Y-L. Novel Polyprenylated Phloroglucinols from Hypericum sampsonii. Molecules. 2014; 19(12):19836-19844. https://doi.org/10.3390/molecules191219836
Chicago/Turabian StyleChen, Jih-Jung, Hong-Jhang Chen, and Yun-Lian Lin. 2014. "Novel Polyprenylated Phloroglucinols from Hypericum sampsonii" Molecules 19, no. 12: 19836-19844. https://doi.org/10.3390/molecules191219836