Field Performance Test of an Air-Cleaner with Photocatalysis-Plasma Synergistic Reactors for Practical and Long-Term Use
Abstract
:1. Introduction


2. Results and Discussion
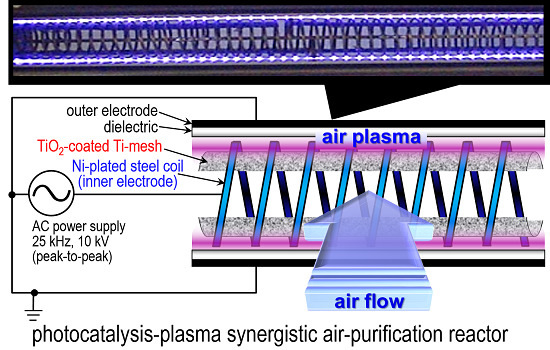
2.1. Evaluations of the Photocatalysis-Plasma Synergistic Air-Cleaner

2.2. Usage of the Smoking Room

2.3. TSP Removal
2.4. TVOC Removal
2.5. Comparison of the Field and Laboratory Tests; The Problems and Future Directions



3. Experimental Section
3.1. Fabrication and Evaluation of the Photocatalysis-Plasma Synergistic Reactor and the Air-Cleaner
3.2. The Evaluation Method of the Air-Purification Activity by the Air-Cleaner in the Smoking Room
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ochiai, T.; Nakata, K.; Murakami, T.; Morito, Y.; Hosokawa, S.; Fujishima, A. Development of an air-purification unit using a photocatalysis-plasma hybrid reactor. Electrochemistry 2011, 79, 838–841. [Google Scholar]
- Ochiai, T.; Fujishima, A. Photoelectrochemical properties of tio2 photocatalyst and its applications for environmental purification. J. Photochem. Photobiol. C 2012, 13, 247–262. [Google Scholar]
- Ochiai, T.; Hayashi, Y.; Ito, M.; Nakata, K.; Murakami, T.; Morito, Y.; Fujishima, A. An effective method for a separation of smoking area by using novel photocatalysis-plasma synergistic air-cleaner. Chem. Eng. J. 2012, 209, 313–317. [Google Scholar]
- Ochiai, T.; Hayashi, Y.; Ichihashi, E.; Machida, T.; Uchida, Y.; Tago, S.; Morito, Y.; Fujishima, A. Development of a coil-shape photocatalysis-plasma synergistic reactor for a practical and long-term usable air-cleaner. Am. J. Anal. Chem. 2014, 5, 467–472. [Google Scholar]
- Chen, X.; Rozak, J.; Lin, J.-C.; Suib, S.L.; Hayashi, Y.; Matsumoto, H. Oxidative decomposition of chlorinated hydrocarbons by glow discharge in pact (plasma and catalyst integrated technologies) reactors. Appl. Catal. A 2001, 219, 25–31. [Google Scholar]
- Ochiai, T.; Hoshi, T.; Slimen, H.; Nakata, K.; Murakami, T.; Tatejima, H.; Koide, Y.; Houas, A.; Horie, T.; Morito, Y.; et al. Fabrication of tio2 nanoparticles impregnated titanium mesh filter and its application for environmental purification unit. Catal. Sci. Technol. 2011, 1, 1324–1327. [Google Scholar]
- Hofer, M.; Penner, D. Thermally stable and photocatalytically active titania for ceramic surfaces. J. Eur. Ceram. Soc. 2011, 31, 2887–2896. [Google Scholar]
- Knudsen, H.N.; Kjaer, U.D.; Nielsen, P.A.; Wolkoff, P. Sensory and chemical characterization of VOC emissions from building products: Impact of concentration and air velocity. Atmos. Environ. 1999, 33, 1217–1230. [Google Scholar]
- Wolkoff, P.; Wilkins, C.K.; Clausen, P.A.; Nielsen, G.D. Organic compounds in office environments—Sensory irritation, odor, measurements and the role of reactive chemistry. Indoor Air 2006, 16, 7–19. [Google Scholar]
- Uhde, E.; Salthammer, T. Impact of reaction products from building materials and furnishings on indoor air quality—A review of recent advances in indoor chemistry. Atmos. Environ. 2007, 41, 3111–3128. [Google Scholar]
- Batterman, S.; Godwin, C.; Jia, C. Long duration tests of room air filters in cigarette smokers’ homes. Environ. Sci. Technol. 2005, 39, 7260–7268. [Google Scholar]
- Cho, M.S.; Ko, H.J.; Kim, D.; Kim, K.Y. On-site application of air cleaner emitting plasma ion to reduce airborne contaminants in pig building. Atmos. Environ. 2012, 63, 276–281. [Google Scholar]
- Molaei Najafabadi, M.; Basirat Tabrizi, H.; Aramesh, A.; Ehteram, M.A. Effects of geometric parameters and electric indexes on performance of a vertical wet electrostatic precipitator. J. Electrost. 2014, 72, 402–411. [Google Scholar]
- Klett, C.; Duten, X.; Tieng, S.; Touchard, S.; Jestin, P.; Hassouni, K.; Vega-González, A. Acetaldehyde removal using an atmospheric non-thermal plasma combined with a packed bed: Role of the adsorption process. J. Hazard. Mater. 2014, 279, 356–364. [Google Scholar]
- Lee, H.; Lee, D.-H.; Song, Y.-H.; Choi, W.C.; Park, Y.-K.; Kim, D.H. Synergistic effect of non-thermal plasma-catalysis hybrid system on methane complete oxidation over Pd-based catalysts. Chem. Eng. J. 2015, 259, 761–770. [Google Scholar]
- Sano, T.; Negishi, N.; Sakai, E.; Matsuzawa, S. Contributions of photocatalytic/catalytic activities of tio2 and γ-al2o3 in nonthermal plasma on oxidation of acetaldehyde and co. J. Mol. Catal. A 2006, 245, 235–241. [Google Scholar]
- Assadi, A.A.; Bouzaza, A.; Merabet, S.; Wolbert, D. Modeling and simulation of vocs removal by nonthermal plasma discharge with photocatalysis in a continuous reactor: Synergetic effect and mass transfer. Chem. Eng. J. 2014, 258, 119–127. [Google Scholar]
- Assadi, A.A.; Bouzaza, A.; Vallet, C.; Wolbert, D. Use of dbd plasma, photocatalysis, and combined dbd plasma/photocatalysis in a continuous annular reactor for isovaleraldehyde elimination—Synergetic effect and byproducts identification. Chem. Eng. J. 2014, 254, 124–132. [Google Scholar]
- Chan, D.; Tischer, S.; Heck, J.; Diehm, C.; Deutschmann, O. Correlation between catalytic activity and catalytic surface area of a pt/al2o3 doc: An experimental and microkinetic modeling study. Appl. Catal. B 2014, 156–157, 153–165. [Google Scholar]
- Caballero-Manrique, G.; Velázquez-Palenzuela, A.; Brillas, E.; Centellas, F.; Garrido, J.A.; Rodríguez, R.M.; Cabot, P.-L. Electrochemical synthesis and characterization of carbon-supported Pt and Pt–Ru nanoparticles with Cu cores for CO and methanol oxidation in polymer electrolyte fuel cells. Int. J. Hydrog. Energy 2014, 39, 12859–12869. [Google Scholar]
- Reshetenko, T.V.; Bethune, K.; Rubio, M.A.; Rocheleau, R. Study of low concentration CO poisoning of Pt anode in a proton exchange membrane fuel cell using spatial electrochemical impedance spectroscopy. J. Power Sources 2014, 269, 344–362. [Google Scholar]
- Sample Availability: Not available.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ochiai, T.; Ichihashi, E.; Nishida, N.; Machida, T.; Uchida, Y.; Hayashi, Y.; Morito, Y.; Fujishima, A. Field Performance Test of an Air-Cleaner with Photocatalysis-Plasma Synergistic Reactors for Practical and Long-Term Use. Molecules 2014, 19, 17424-17434. https://doi.org/10.3390/molecules191117424
Ochiai T, Ichihashi E, Nishida N, Machida T, Uchida Y, Hayashi Y, Morito Y, Fujishima A. Field Performance Test of an Air-Cleaner with Photocatalysis-Plasma Synergistic Reactors for Practical and Long-Term Use. Molecules. 2014; 19(11):17424-17434. https://doi.org/10.3390/molecules191117424
Chicago/Turabian StyleOchiai, Tsuyoshi, Erina Ichihashi, Naoki Nishida, Tadashi Machida, Yoshitsugu Uchida, Yuji Hayashi, Yuko Morito, and Akira Fujishima. 2014. "Field Performance Test of an Air-Cleaner with Photocatalysis-Plasma Synergistic Reactors for Practical and Long-Term Use" Molecules 19, no. 11: 17424-17434. https://doi.org/10.3390/molecules191117424