The K2CO3–CaCO3–MgCO3 System at 6 GPa: Implications for Diamond Forming Carbonatitic Melts
Abstract
:1. Introduction
2. Materials and Methods
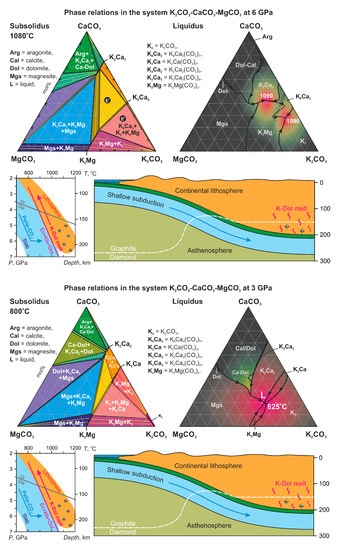
3. Experimental Results
24.71K2(Mg0.6Ca0.4)(CO3)2 (K2Mg) = [40K2CO3∙60(Ca0.70Mg0.30)CO3] (LE1)
(K2Mg) = [62K2CO3∙38(Ca0.73Mg0.27)CO3] (LE2)
4. Discussion
4.1. Comparison with the Previous Study
4.2. Effect of Pressure
4.3. Comparison with the Na-Bearing System
4.4. Mutual Solubility of K2Ca(CO3)2 and K2Mg(CO3)2
4.5. Variety of Carbonates in Diamonds and Their Origin
4.6. Thermal Stability of Carbonatitic Melts vs. Alkalinity
4.7. Daughter Carbonate Minerals, Which Can Be Expected in Diamond Inclusions
4.8. Comparison with Carbonated Pelite-Derived Melts
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Nomenclature
| Arg = aragonite, | K2 = K2CO3, |
| Cal = calcite, | K8Ca3 = K8Ca3(CO3)7, |
| Mg-Cal = Mg-bearing calcite, | K2Ca = K2Ca(CO3)2, |
| Ca-Dol = Ca-rich dolomite, | K2Ca2 = K2Ca2(CO3)3, |
| Dol = dolomite, | K2Ca3 = K2Ca3(CO3)4, |
| Mgs = magnesite, | K2Mg = K2Mg(CO3)2. |
| L = liquid, |
References
- Pyle, J.M.; Haggerty, S.E. Silicate-carbonate liquid immiscibility in upper-mantle eclogites: implications for natrosilicic and carbonatitic conjugate melts. Geochim. Cosmochim. Acta 1994, 58, 2997–3011. [Google Scholar] [CrossRef]
- Kogarko, L.N.; Henderson, C.M.B.; Pacheco, H. Primary Ca-rich carbonatite magma and carbonate-silicate-sulphide liquid immiscibility in the upper mantle. Contrib. Mineral. Petrol. 1995, 121, 267–274. [Google Scholar] [CrossRef]
- Ionov, D. Trace element composition of mantle-derived carbonates and coexisting phases in peridotite xenoliths from alkali basalts. J. Petrol. 1998, 39, 1931–1941. [Google Scholar] [CrossRef]
- Nikolenko, E.I.; Sharygin, I.S.; Alifirova, T.A.; Korsakov, A.V.; Zelenovskiy, P.S.; Shur, V.Y. Graphite-bearing mineral assemblages in the mantle beneath Central Aldan superterrane of North Asian craton: combined confocal micro-Raman and electron microprobe characterization. J. Raman Spectrosc. 2017, 48, 1597–1605. [Google Scholar] [CrossRef]
- Rezvukhin, D.I.; Malkovets, V.G.; Sharygin, I.S.; Tretiakova, I.G.; Griffin, W.L.; O’Reilly, S.Y. Inclusions of crichtonite-group minerals in Cr-pyropes from the Internatsionalnaya kimberlite pipe, Siberian Craton: Crystal chemistry, parageneses and relationships to mantle metasomatism. Lithos 2018, 308, 181–195. [Google Scholar] [CrossRef]
- Meyer, H.O.A.; McCallum, M.E. Mineral inclusions in diamonds from the Sloan kimberlites, Colorado. J. Geology 1986, 94, 600–612. [Google Scholar] [CrossRef]
- Bulanova, G.P.; Pavlova, L.P. Magnesite peridotite assemblage in diamond from the Mir pipe. Dokl. Akad. Nauk SSSR 1987, 295, 1452–1456. [Google Scholar]
- Wang, A.; Pasteris, J.D.; Meyer, H.O.A.; DeleDuboi, M.L. Magnesite-bearing inclusion assemblage in natural diamond. Earth Planet. Sci. Lett. 1996, 141, 293–306. [Google Scholar] [CrossRef]
- Stachel, T.; Harris, J.W.; Brey, G.P. Rare and unusual mineral inclusions in diamonds from Mwadui, Tanzania. Contrib. Mineral. Petrol. 1998, 132, 34–47. [Google Scholar] [CrossRef]
- Brenker, F.E.; Vollmer, C.; Vincze, L.; Vekemans, B.; Szymanski, A.; Janssens, K.; Szaloki, I.; Nasdala, L.; Joswig, W.; Kaminsky, F. Carbonates from the lower part of transition zone or even the lower mantle. Earth Planet. Sci. Lett. 2007, 260, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Sobolev, N.V.; Logvinova, A.M.; Efimova, E.S. Syngenetic phlogopite inclusions in kimberlite-hosted diamonds: implications for role of volatiles in diamond formation. Russ. Geol. Geophys. 2009, 50, 1234–1248. [Google Scholar] [CrossRef]
- Logvinova, A.M.; Wirth, R.; Zedgenizov, D.A.; Taylor, L.A. Carbonate–Silicate–Sulfide Polyphase Inclusion in Diamond from the Komsomolskaya Kimberlite Pipe, Yakutia. Geochem. Int. 2018, 56, 283–291. [Google Scholar] [CrossRef]
- Zedgenizov, D.A.; Shatskiy, A.; Ragozin, A.L.; Kagi, H.; Shatsky, V.S. Merwinite in diamond from São Luis, Brazil: A new mineral of the Ca-rich mantle environment. Am. Mineral. 2014, 99, 547–550. [Google Scholar] [CrossRef]
- Sobolev, N.V.; Shatsky, V.S. Diamond inclusions in garnets from metamorphic rocks: a new environment for diamond formation. Nature 1990, 343, 742–746. [Google Scholar] [CrossRef]
- Shatsky, V.S.; Sobolev, N.V.; Vavilov, M.A. Diamond-bearing metamorphic rocks from the Kokchetav massif (Northern Kazakhstan). In Ultrahigh Pressure Metamorphism; Coleman, R.G., Wang, X., Eds.; Cambridge University Press: Cambridge, UK, 1995; pp. 427–455. [Google Scholar]
- Dobrzhinetskaya, L.F.; Wirth, R.; Green, H.W. Nanometric inclusions of carbonates in Kokchetav diamonds from Kazakhstan: A new constraint for the depth of metamorphic diamond crystallization. Earth Planet. Sci. Lett. 2006, 243, 85–93. [Google Scholar] [CrossRef]
- Korsakov, A.V.; Hermann, J. Silicate and carbonate melt inclusions associated with diamonds in deeply subducted carbonate rocks. Earth Planet. Sci. Lett. 2006, 241, 104–118. [Google Scholar] [CrossRef]
- Navon, O.; Hutcheon, I.; Rossman, G.; Wasserburg, G. Mantle-derived fluids in diamond micro-inclusions. Nature 1988, 335, 784–789. [Google Scholar] [CrossRef]
- Schrauder, M.; Navon, O. Hydrous and carbonatitic mantle fluids in fibrous diamonds from Jwaneng, Botswana. Geochim. Cosmochim. Acta 1994, 58, 761–771. [Google Scholar] [CrossRef]
- Zedgenizov, D.A.; Ragozin, A.L.; Shatsky, V.S.; Araujo, D.; Griffin, W.L.; Kagi, H. Mg and Fe-rich carbonate-silicate high-density fluids in cuboid diamonds from the Internationalnaya kimberlite pipe (Yakutia). Lithos 2009, 112, 638–647. [Google Scholar] [CrossRef]
- Klein-BenDavid, O.; Logvinova, A.M.; Schrauder, M.; Spetius, Z.V.; Weiss, Y.; Hauri, E.H.; Kaminsky, F.V.; Sobolev, N.V.; Navon, O. High-Mg carbonatitic micro inclusions in some Yakutian diamonds—A new type of diamond-forming fluid. Lithos 2009, 112, 648–659. [Google Scholar] [CrossRef]
- Weiss, Y.; Kessel, R.; Griffin, W.L.; Kiflawi, I.; Klein-BenDavid, O.; Bell, D.R.; Harris, J.W.; Navon, O. A new model for the evolution of diamond-forming fluids: Evidence from microinclusion-bearing diamonds from Kankan, Guinea. Lithos 2009, 112, 660–674. [Google Scholar] [CrossRef]
- Skuzovatov, S.; Zedgenizov, D.; Howell, D.; Griffin, W.L. Various growth environments of cloudy diamonds from the Malobotuobia kimberlite field (Siberian craton). Lithos 2016, 265, 96–107. [Google Scholar] [CrossRef]
- Logvinova, A.M.; Wirth, R.; Tomilenko, A.A.; Afanas’ev, V.P.; Sobolev, N.V. The phase composition of crystal-fluid nanoinclusions in alluvial diamonds in the northeastern Siberian Platform. Russ. Geol. Geophys. 2011, 52, 1286–1297. [Google Scholar] [CrossRef]
- Tomlinson, E.L.; Jones, A.P.; Harris, J.W. Co-existing fluid and silicate inclusions in mantle diamond. Earth Planet. Sci. Lett. 2006, 250, 581–595. [Google Scholar] [CrossRef]
- Navon, O. High internal pressure in diamond fluid inclusions determined by infrared absorption. Nature 1991, 353, 746–748. [Google Scholar] [CrossRef]
- Logvinova, A.M.; Shatskiy, A.; Wirth, R.; Ugap’eva, S.S.; Sobolev, N.V. Carbonatite melt in type Ia gem diamond. Lithos 2019, 342-343, 463–467. [Google Scholar] [CrossRef]
- Jablon, B.M.; Navon, O. Most diamonds were created equal. Earth Planet. Sci. Lett. 2016, 443, 41–47. [Google Scholar] [CrossRef]
- Tsuno, K.; Dasgupta, R.; Danielson, L.; Righter, K. Flux of carbonate melt from deeply subducted pelitic sediments: Geophysical and geochemical implications for the source of Central American volcanic arc. Geophys. Res. Lett. 2012, 39. [Google Scholar] [CrossRef] [Green Version]
- Grassi, D.; Schmidt, M.W. Melting of carbonated pelites at 8–13 GPa: Generating K-rich carbonatites for mantle metasomatism. Contrib. Mineral. Petrol. 2011, 162, 169–191. [Google Scholar] [CrossRef]
- Shatskiy, A.; Arefiev, A.V.; Podborodnikov, I.V.; Litasov, K.D. Origin of K-rich diamond-forming immiscible melts and CO2 fluid via partial melting of carbonated pelites at a depth of 180-200 km. Gondwana Res. 2019, 75, 154–171. [Google Scholar] [CrossRef]
- Litasov, K.D.; Shatskiy, A.; Ohtani, E.; Yaxley, G.M. The solidus of alkaline carbonatite in the deep mantle. Geology 2013, 41, 79–82. [Google Scholar] [CrossRef]
- Shatskiy, A.; Litasov, K.D.; Palyanov, Y.N.; Ohtani, E. Phase relations on the K2CO3-CaCO3-MgCO3 join at 6 GPa and 900–1400° C: Implications for incipient melting in carbonated mantle domains. Am. Mineral. 2016, 101, 437–447. [Google Scholar] [CrossRef]
- Shatskiy, A.; Litasov, K.D.; Terasaki, H.; Katsura, T.; Ohtani, E. Performance of semi-sintered ceramics as pressure-transmitting media up to 30 GPa. High Press. Res. 2010, 30, 443–450. [Google Scholar] [CrossRef]
- Shatskiy, A.; Podborodnikov, I.V.; Arefiev, A.V.; Minin, D.A.; Chanyshev, A.D.; Litasov, K.D. Revision of the CaCO3–MgCO3 phase diagram at 3 and 6 GPa. Am. Mineral. 2018, 103, 441–452. [Google Scholar] [CrossRef]
- Shatskiy, A.; Sharygin, I.S.; Gavryushkin, P.N.; Litasov, K.D.; Borzdov, Y.M.; Shcherbakova, A.V.; Higo, Y.; Funakoshi, K.-I.; Palyanov, Y.N.; Ohtani, E. The system K2CO3-MgCO3 at 6 GPa and 900–1450 °C. Am. Mineral. 2013, 98, 1593–1603. [Google Scholar] [CrossRef]
- Arefiev, A.V.; Shatskiy, A.; Podborodnikov, I.V.; Behtenova, A.; Litasov, K.D. The system K2CO3–CaCO3–MgCO3 at 3 GPa: Implications for carbonatite melt compositions in the subcontinental lithospheric mantle. Minerals 2019, 9, 296. [Google Scholar] [CrossRef]
- Shatskiy, A.; Borzdov, Y.M.; Litasov, K.D.; Sharygin, I.S.; Palyanov, Y.N.; Ohtani, E. Phase relationships in the system K2CO3-CaCO3 at 6 GPa and 900–1450 °C. Am. Mineral. 2015, 100, 223–232. [Google Scholar] [CrossRef]
- Arefiev, A.V.; Shatskiy, A.; Podborodnikov, I.V.; Rashchenko, S.V.; Chanyshev, A.D.; Litasov, K.D. The system K2CO3-CaCO3 at 3 GPa: Link between phase relations and variety of K-Ca double carbonates at ≤ 0.1 and 6 GPa. Phys. Chem. Miner. 2019, 46, 229–244. [Google Scholar] [CrossRef]
- Arefiev, A.V.; Shatskiy, A.; Podborodnikov, I.V.; Litasov, K.D. Melting and subsolidus phase relations in the system K2CO3-MgCO3 at 3 GPa. High Press. Res. 2018, 38, 422–439. [Google Scholar] [CrossRef]
- Cooper, A.F.; Gittins, J.; Tuttle, O.F. The system Na2CO3-K2CO3-CaCO3 at 1 kilobar and its significance in carbonatite petrogenesis. Am. J. Sci. 1975, 275, 534–560. [Google Scholar] [CrossRef]
- Ragone, S.E.; Datta, R.K.; Roy, D.M.; Tuttle, O.F. The system potassium carbonate-magnesium carbonate. J. Phys. Chem. 1966, 70, 3360–3361. [Google Scholar] [CrossRef]
- Arefiev, A.V.; Podborodnikov, I.V.; Shatskiy, A.F.; Litasov, K.D. Synthesis and Raman spectra of K-Ca double carbonates: K2Ca(CO3)2 bütschliite, fairchildite and K2Ca2(CO3)3 at 1 atm. Geochem. Int. 2019, 57, 981–987. [Google Scholar] [CrossRef]
- Podborodnikov, I.V.; Shatskiy, A.; Arefiev, A.V.; Bekhtenova, A.; Litasov, K.D. New data on the system Na2CO3–CaCO3–MgCO3 at 6 GPa with implications to the composition and stability of carbonatite melts at the base of continental lithosphere. Chem. Geol. 2019, 515, 50–60. [Google Scholar] [CrossRef]
- Podborodnikov, I.V.; Shatskiy, A.; Arefiev, A.V.; Litasov, K.D. Phase relations in the system Na2CO3–CaCO3–MgCO3 at 3 GPa with implications for carbonatite genesis and evolution. Lithos 2019, 330–331, 74–89. [Google Scholar] [CrossRef]
- Eitel, W.; Skaliks, W. Ueber einige doppelcarbonate der alkalien und erdalkalien. Z. Anorg. Allg. Chem. 1929, 183, 263–286. [Google Scholar] [CrossRef]
- Brey, G.P.; Bulatov, V.K.; Girnis, A.V. Melting of K-rich carbonated peridotite at 6-10 GPa and the stability of K-phases in the upper mantle. Chem. Geol. 2011, 281, 333–342. [Google Scholar] [CrossRef]
- Pabst, A. Synthesis, properties, and structure of K2Ca(CO3)2, buetschliite. Am. Mineral. 1974, 59, 353–358. [Google Scholar]
- Hesse, K.-F.; Simons, B. Crystalstructure of synthetic K2Mg(CO3)2. Z. Krist. 1982, 161, 289–292. [Google Scholar] [CrossRef]
- Golubkova, A.; Merlini, M.; Schmidt, M.W. Crystal structure, high-pressure, and high-temperature behavior of carbonates in the K2Mg(CO3)2–Na2Mg(CO3)2 join. Am. Mineral. 2015, 100, 2458–2467. [Google Scholar] [CrossRef]
- Logvinova, A.; Zedgenizov, D.; Wirth, R. Specific Multiphase assemblages of carbonatitic and Al-rich silicic diamond-forming fluids/melts: TEM observation of micro inclusions in cuboid diamonds from the placers of northeastern Siberian Craton. Minerals 2019, 9, 50. [Google Scholar] [CrossRef]
- Stachel, T.; Harris, J.W.; Brey, G.P.; Joswig, W. Kankan diamonds (Guinea) II: Lower mantle inclusion parageneses. Contrib. Mineral. Petrol. 2000, 140, 16–27. [Google Scholar] [CrossRef]
- Kaminsky, F.; Wirth, R.; Matsyuk, S.; Schreiber, A.; Thomas, R. Nyerereite and nahcolite inclusions in diamond: Evidence for lower-mantle carbonatitic magmas. Mineral. Mag. 2009, 73, 797–816. [Google Scholar] [CrossRef]
- Smith, E.M.; Kopylova, M.G.; Dubrovinsky, L.; Navon, O.; Ryder, J.; Tomlinson, E.L. Transmission X-ray diffraction as a new tool for diamond fluid inclusion studies. Mineral. Mag. 2011, 75, 2657–2675. [Google Scholar] [CrossRef]
- Kaminsky, F.V.; Wirth, R.; Schreiber, A. Carbonatitic inclusions in deep mantle diamond from Juina, Brazil: New minerals in the carbonate-halide association. Can. Mineral. 2013, 51, 669–688. [Google Scholar] [CrossRef]
- Stachel, T.; Luth, R.W. Diamond formation—Where, when and how? Lithos 2015, 220, 200–220. [Google Scholar] [CrossRef]
- Pal’yanov, Y.N.; Sokol, A.G.; Borzdov, Y.M.; Khokhryakov, A.F.; Sobolev, N.V. Diamond formation from mantle carbonate fluids. Nature 1999, 400, 417–418. [Google Scholar] [CrossRef]
- Palyanov, Y.N.; Bataleva, Y.V.; Sokol, A.G.; Borzdov, Y.M.; Kupriyanov, I.N.; Reutsky, V.N.; Sobolev, N.V. Mantle–slab interaction and redox mechanism of diamond formation. Proc. Natl. Acad. Sci. USA 2013, 110, 20408–20413. [Google Scholar] [CrossRef]
- Girnis, A.V.; Brey, G.P.; Bulatov, V.K.; Höfer, H.E.; Woodland, A.B. Graphite to diamond transformation during sediment–peridotite interaction at 7.5 and 10.5 GPa. Lithos 2018, 310, 302–313. [Google Scholar] [CrossRef]
- Wang, Y.; Kanda, H. Growth of HPHT diamonds in alkali halides: possible effects of oxygen contamination. Diam. Relat. Mater. 1998, 7, 57–63. [Google Scholar] [CrossRef]
- Arima, M.; Nakayama, K.; Akaishi, M.; Yamaoka, S.; Kanda, H. Crystallization of diamond from a silicate melt of kimberlite composition in high-pressure and high-temperature experiments. Geology 1993, 21, 968–970. [Google Scholar] [CrossRef]
- Akaishi, M. New nonmetallic catalysts for the synthesis of high-pressure, high-temperature diamond. Diam. Relat. Mater. 1993, 2, 183–189. [Google Scholar] [CrossRef]
- Palyanov, Y.N.; Sokol, A.G. The effect of composition of mantle fluids/melts on diamond formation processes. Lithos 2009, 112, 690–700. [Google Scholar] [CrossRef]
- Katsura, T.; Yoneda, A.; Yamazaki, D.; Yoshino, T.; Ito, E. Adiabatic temperature profile in the mantle. Phys. Earth Planet. Inter. 2010, 183, 212–218. [Google Scholar] [CrossRef]
- Kennedy, C.S.; Kennedy, G.C. The equilibrium boundary between graphite and diamond. J. Geophys. Res. 1976, 81, 2467–2470. [Google Scholar] [CrossRef]
- Shatskiy, A.; Litasov, K.D.; Sharygin, I.S.; Ohtani, E. Composition of primary kimberlite melt in a garnet lherzolite mantle source: Constraints from melting phase relations in anhydrous Udachnaya-East kimberlite with variable CO2 content at 6.5 GPa. Gondwana Res. 2017, 45, 208–227. [Google Scholar] [CrossRef]
- Kavanagh, J.L.; Sparks, R.S.J. Temperature changes in ascending kimberlite magma. Earth Planet. Sci. Lett. 2009, 286, 404–413. [Google Scholar] [CrossRef]
- Pollack, H.N.; Chapman, D.S. On the regional variation of heat flow, geotherms, and lithospheric thickness. Tectonophysics 1977, 38, 279–296. [Google Scholar] [CrossRef] [Green Version]
- Zedgenizov, D.A.; Logvinova, A.M.; Shatskii, V.S.; Sobolev, N.V. Inclusions in microdiamonds from some kimberlite diatremes of Yakutia. Dokl. Earth Sci. 1998, 359, 204–208. [Google Scholar]
- Klein-BenDavid, O.; Izraeli, E.S.; Hauri, E.; Navon, O. Mantle fluid evolution—A tale of one diamond. Lithos 2004, 77, 243–253. [Google Scholar] [CrossRef]
- Klein-BenDavid, O.; Wirth, R.; Navon, O. TEM imaging and analysis of micro inclusions in diamonds: A close look at diamond-growing fluids. Am. Mineral. 2006, 91, 353–365. [Google Scholar] [CrossRef]
- Klein-BenDavid, O.; Izraeli, E.S.; Hauri, E.; Navon, O. Fluid inclusions in diamonds from the Diavik mine, Canada and the evolution of diamond-forming fluids. Geochim. Cosmochim. Acta 2007, 71, 723–744. [Google Scholar] [CrossRef]
- Zedgenizov, D.A.; Rege, S.; Griffin, W.L.; Kagi, H.; Shatsky, V.S. Composition of trapped fluids in cuboid fibrous diamonds from the Udachnaya kimberlite: LAM-ICPMS analysis. Chem. Geol. 2007, 240, 151–162. [Google Scholar] [CrossRef]
- Skuzovatov, S.Y.; Zedgenizov, D.A.; Shatsky, V.S.; Ragozin, A.L.; Kuper, K.E. Composition of cloudy micro inclusions in octahedral diamonds from the Internatsional’naya kimberlite pipe (Yakutia). Russ. Geol. Geophys. 2011, 52, 85–96. [Google Scholar] [CrossRef]
- Zedgenizov, D.A.; Ragozin, A.L.; Shatsky, V.S.; Araujo, D.; Griffin, W.L. Fibrous diamonds from the placers of the northeastern Siberian Platform: carbonate and silicate crystallization media. Russ. Geol. Geophys. 2011, 52, 1298–1309. [Google Scholar] [CrossRef]
- Smith, E.M.; Kopylova, M.G.; Nowell, G.M.; Pearson, D.G.; Ryder, J. Archean mantle fluids preserved in fibrous diamonds from Wawa, Superior craton. Geology 2012, 40, 1071–1074. [Google Scholar] [CrossRef]
- Weiss, Y.; Kiflawi, I.; Davies, N.; Navon, O. High-density fluids and the growth of monocrystalline diamonds. Geochim. Cosmochim. Acta 2014, 141, 145–159. [Google Scholar] [CrossRef]
- Shatskiy, A.; Borzdov, Y.M.; Litasov, K.D.; Kupriyanov, I.N.; Ohtani, E.; Palyanov, Y.N. Phase relations in the system FeCO3-CaCO3 at 6 GPa and 900–1700 °C and its relation to the system CaCO3-FeCO3-MgCO3. Am. Mineral. 2014, 99, 773–785. [Google Scholar] [CrossRef]
- Shatskiy, A.; Litasov, K.D.; Ohtani, E.; Borzdov, Y.M.; Khmelnicov, A.I.; Palyanov, Y.N. Phase relations in the K2CO3-FeCO3 and MgCO3-FeCO3 systems at 6 GPa and 900–1700 °C. Eur. J. Mineral. 2015, 27, 487–499. [Google Scholar] [CrossRef]
- Tychkov, N.S.; Agashev, A.M.; Malygina, E.V.; Nikolenko, E.I.; Pokhilenko, N.P. Thermal perturbations in the lithospheric mantle as evidenced from PT equilibrium conditions of xenoliths from the Udachnaya kimberlite pipe. Dokl. Earth Sci. 2014, 454, 84–88. [Google Scholar] [CrossRef]
- Agashev, A.M.; Pokhilenko, L.N.; Pokhilenko, N.P.; Shchukina, E.V. Geochemistry of eclogite xenoliths from the Udachnaya Kimberlite Pipe: Section of ancient oceanic crust sampled. Lithos 2018, 314–315, 187–200. [Google Scholar] [CrossRef]
- Boyd, F.R.; Nixon, P.H. Origins of the ultramafic nodules from some kimberlites of northern Lesotho and the Monastery Mine, South Africa. Phys. Chem. Earth 1975, 9, 431–454. [Google Scholar] [CrossRef]
- Green, H.W.; Gueguen, Y. Origin of kimberlite pipes by diapiric upwelling in the upper mantle. Nature 1974, 249, 617–620. [Google Scholar] [CrossRef]
- Agashev, A.M.; Pokhilenko, N.R.; Takazawa, E.; McDonald, J.A.; Vavilov, M.A.; Watanabe, I.; Sobolev, N.V. Primary melting sequence of a deep (>250 km) lithospheric mantle as recorded in the geochemistry of kimberlite-carbonatite assemblages, Snap Lake dyke system, Canada. Chem. Geol. 2008, 255, 317–328. [Google Scholar] [CrossRef]
- Yaxley, G.M.; Brey, G.P. Phase relations of carbonate-bearing eclogite assemblages from 2.5 to 5.5 GPa: Implications for petrogenesis of carbonatites. Contrib. Mineral. Petrol. 2004, 146, 606–619. [Google Scholar] [CrossRef]
- Dasgupta, R.; Hirschmann, M.M. Effect of variable carbonate concentration on the solidus of mantle peridotite. Am. Mineral. 2007, 92, 370–379. [Google Scholar] [CrossRef]
- Brey, G.P.; Bulatov, V.K.; Girnis, A.V.; Lahaye, Y. Experimental melting of carbonated peridotite at 6-10 GPa. J. Petrol. 2008, 49, 797–821. [Google Scholar] [CrossRef]











© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arefiev, A.V.; Shatskiy, A.; Podborodnikov, I.V.; Litasov, K.D. The K2CO3–CaCO3–MgCO3 System at 6 GPa: Implications for Diamond Forming Carbonatitic Melts. Minerals 2019, 9, 558. https://doi.org/10.3390/min9090558
Arefiev AV, Shatskiy A, Podborodnikov IV, Litasov KD. The K2CO3–CaCO3–MgCO3 System at 6 GPa: Implications for Diamond Forming Carbonatitic Melts. Minerals. 2019; 9(9):558. https://doi.org/10.3390/min9090558
Chicago/Turabian StyleArefiev, Anton V., Anton Shatskiy, Ivan V. Podborodnikov, and Konstantin D. Litasov. 2019. "The K2CO3–CaCO3–MgCO3 System at 6 GPa: Implications for Diamond Forming Carbonatitic Melts" Minerals 9, no. 9: 558. https://doi.org/10.3390/min9090558