Representation of Kinetics Models in Batch Flotation as Distributed First-Order Reactions
Abstract
:1. Introduction
1.1. Background
1.2. Kinetic Models in Flotation
1.2.1. First-Order Models
1.2.2. nth-Order Models
1.2.3. Other Model Structures
2. Materials and Methods
2.1. First-Order Representation
2.2. Flotation Tests
3. Results
3.1. nth-Order Models with Deterministic Rate Constants Represented as First-Order Models with Gamma f(k)s
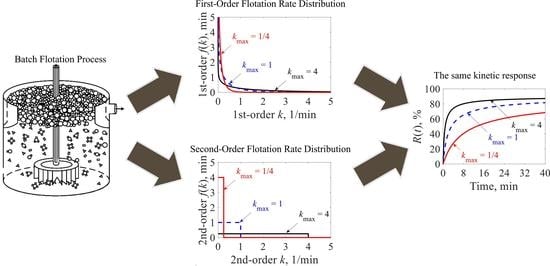
3.2. Second-Order Model with Rectangular f(k) Represented as a Distributed First-Order Reaction
3.3. Rosin-Rammler Model Represented as a Distributed First-Order Model
3.4. Fractional Kinetics as Distributed First-Order Reactions
4. Discussion
5. Conclusions
- The nth-order reactions with determinist rate constants can be represented as first-order reactions with Gamma f(k)s. Low reaction orders indicate approximately deterministic rate constants, whereas high reaction orders indicate J-shaped f(k)s. The latter implies a high presence of slow-floating components (k → 0).
- The second-order reaction with Rectangular distribution of rate constants can be represented as a first-order reaction with f(k) = 1/kmax∙E1(k/kmax). These first-order f(k)s are always reverse J-shaped distributions, indicating high concentrations of slow rate constants and then sustained increasing trends in R(t).
- The Rosin–Rammler model has a first-order representation for aRR ≤ 1 (stretched exponentials). In this case, the typically unimodal f(k)s only presented rate constants close to zero with aRR → 0. For aRR > 1 (compressed exponentials), the Rosin–Rammler model does not have a first-order representation.
- The Fractional kinetics can be represented as a first-order reaction for α ≤ 1. Although the f(k)s approached to deterministic rate constants as α → 1, slow rate fractions were observed up to moderate high α values. For α > 1, the Fractional approach does not have physical meaning in flotation.
Author Contributions
Funding
Conflicts of Interest
References
- Garcia-Zuñiga, H. La recuperación por flotación es una función exponencial del tiempo. Boletín Minero, Sociedad Nacional de Minería 1935, 47, 83–86. [Google Scholar]
- Schuhmann, R., Jr. Flotation Kinetics. I. Methods for steady-state study of flotation problems. J. Phys. Chem. 1942, 46, 891–902. [Google Scholar] [CrossRef]
- Arbiter, N. Flotation rates and flotation efficiency. Trans. Am. Inst. Min. Metall. Eng. 1951, 190, 791–796. [Google Scholar]
- Kelsall, D.F. Application of probability assessment of flotation systems. Trans. Inst. Min. Metall. 1961, 70, 191–204. [Google Scholar]
- Imaizumi, T.; Inoue, T. Kinetic consideration of froth flotation. In Proceedings of the Sixth International Mineral Processing Congress, Cannes, France, 26 May–2 June 1963; pp. 581–593. [Google Scholar]
- Ahmed, M.M. Discrimination of different models in the flotation of Maghara coal. Miner. Process. Extr. Metall. 2004, 113, 103–110. [Google Scholar] [CrossRef]
- Horst, W. Scale-up relationships in spodumene flotation. Min. Eng. 1958, 10, 1182–1185. [Google Scholar]
- Sutherland, K.L. Physical chemistry of flotation. XI. Kinetics of the flotation process. J. Phys. Chem. 1948, 52, 394–425. [Google Scholar] [CrossRef]
- Woodburn, E.T.; Loveday, B.K. The effect of variable residence time on the performance of a flotation system. J. S. Afr. Inst. Min. Metall. 1965, 65, 612–628. [Google Scholar]
- Trahar, W.J. A rational interpretation of the role of particle size in flotation. Int. J. Miner. Process. 1981, 8, 289–327. [Google Scholar] [CrossRef]
- Morris, T. Measurement and evaluation of the rate of flotation as a function of particle size. Min. Eng. 1952, 4, 794–798. [Google Scholar]
- Gaudin, A.M.; Groh, J.O.; Henderson, H.B. Effect of particle size on flotation. Am. Inst. Min. Metall. Eng. 1931, 414, 3–23. [Google Scholar]
- Gaudin, A.M.; Schuhmann, R., Jr.; Schlechten, A.W. Flotation Kinetics. II. The Effect of Size on the Behavior of Galena Particles. J. Phys. Chem. 1942, 46, 902–910. [Google Scholar] [CrossRef]
- Sutherland, D.N. Batch flotation behaviour of composite particles. Miner. Eng. 1989, 2, 351–367. [Google Scholar] [CrossRef]
- Bartlett, D.R.; Mular, A.L. Dependence of flotation rate on particle size and fractional mineral content. Int. J. Miner. Process. 1974, 1, 277–286. [Google Scholar] [CrossRef]
- Welsby, S.D.D.; Vianna, S.M.S.M.; Franzidis, J.-P. Assigning physical significance to floatability components. Int. J. Miner. Process. 2010, 97, 59–67. [Google Scholar] [CrossRef]
- Jameson, G.J. The effect of surface liberation and particle size on flotation rate constants. Miner. Eng. 2012, 36–38, 132–137. [Google Scholar] [CrossRef]
- Huber-Panu, I.; Ene-Danalache, E.; Cojocariu, D.G. Mathematical models of batch and continuous flotation. In Flotation—A. M. Gaudin Memorial; American Institute of Mining, Metallurgical, and Petroleum Engineers, Inc.: New York, NY, USA, 1976; Volume 2, pp. 675–724. [Google Scholar]
- Dowling, E.C.; Klimpel, R.R.; Aplan, F.F. Model discrimination in the flotation of a porphyry copper ore. Miner. Metall. Process. 1985, 2, 87–101. [Google Scholar] [CrossRef]
- Diao, J.; Fuerstenau, D.; Hanson, J. Kinetics of coal flotation. In Proceedings of the SME-AIME Annual Meeting, Phoenix, AZ, USA, 21–24 February 2016. [Google Scholar]
- Harris, C.; Chakravarti, A. Semi-batch froth flotation kinetics: Species distribution analysis. Trans. AIME 1970, 247, 162–172. [Google Scholar]
- Chander, S.; Polat, M. Flotation Kinetics: Methods for Estimating Distribution of Rate Constants. In Proceedings of the International Mineral Processing Congress (XIX IMPC), San Francisco, CA, USA, 22–27 October 1995; pp. 105–111. [Google Scholar]
- Ferreira, J.P.; Loveday, B.K. An improved model for simulation of flotation circuits. Miner. Eng. 2000, 13, 1441–1453. [Google Scholar] [CrossRef]
- Yianatos, J.; Bergh, L.; Vinnett, L.; Contreras, F.; Díaz, F. Flotation rate distribution in the collection zone of industrial cells. Miner. Eng. 2010, 23, 1030–1035. [Google Scholar] [CrossRef]
- Klimpel, R.R. Selection of chemical reagents for flotation. In Mineral Processing Plant Design; Mular, A.L., Bhappu, R.B., Eds.; SME-AIME: New York, NY, USA, 1980; Volume 2, pp. 907–934. [Google Scholar]
- Bahrami, A.; Ghorbani, Y.; Hosseini, M.R.; Kazemi, F.; Abdollahi, M.; Danesh, A. Combined Effect of Operating Parameters on Separation Efficiency and Kinetics of Copper Flotation. Min. Metall. Explor. 2019, 36, 409–421. [Google Scholar] [CrossRef] [Green Version]
- Bu, X.; Xie, G.; Chen, Y.; Ni, C. The Order of Kinetic Models in Coal Fines Flotation. Int. J. Coal Prep. Util. 2017, 37, 113–123. [Google Scholar] [CrossRef]
- Chaves, A.P.; Ruiz, A.S. Considerations on the Kinetics of Froth Flotation of Ultrafine Coal Contained in Tailings. Int. J. Coal Prep. Util. 2009, 29, 289–297. [Google Scholar] [CrossRef]
- Ek, C. Flotation Kinetics. In Innovations in Flotation Technology; Mavros, P., Matis, K.A., Eds.; Springer: Dordrecht, The Netherlands, 1992; pp. 183–210. [Google Scholar]
- Ni, C.; Xie, G.; Jin, M.; Peng, Y.; Xia, W. The difference in flotation kinetics of various size fractions of bituminous coal between rougher and cleaner flotation processes. Powder Technol. 2016, 292, 210–216. [Google Scholar] [CrossRef]
- Yuan, X.M.; Palsson, B.I.; Forssberg, K.S.E. Statistical interpretation of flotation kinetics for a complex sulphide ore. Miner. Eng. 1996, 9, 429–442. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, J.; Cao, Y.; Wang, Y. Effects of particle size on lignite reverse flotation kinetics in the presence of sodium chloride. Powder Technol. 2013, 246, 658–663. [Google Scholar] [CrossRef]
- Yang, X.; Huang, X.; Qiu, T. Activation-flotation kinetics of depressed marmatite and chalcopyrite in cyanidation tailings using sodium hypochlorite as activator. Miner. Metall. Process. 2016, 33, 131–136. [Google Scholar] [CrossRef]
- Tijsseling, L.T.; Dehaine, Q.; Rollinson, G.K.; Glass, H.J. Mineralogical Prediction of Flotation Performance for a Sediment-Hosted Copper–Cobalt Sulphide Ore. Minerals 2020, 10, 474. [Google Scholar] [CrossRef]
- Bu, X.; Xie, G.; Peng, Y.; Ge, L.; Ni, C. Kinetics of flotation. Order of process, rate constant distribution and ultimate recovery. Physicochem. Probl. Miner. Process. 2017, 53, 342–365. [Google Scholar]
- Tewari, S.N.; Biswas, A.K. Flotation kinetics for calcite in a semi-batch system. J. Appl. Chem. 1969, 19, 173–177. [Google Scholar] [CrossRef]
- Brożek, M.; Młynarczykowska, A. Analysis of kinetics models of batch flotation. Physicochem. Probl. Miner. Process. 2007, 41, 51–65. [Google Scholar]
- de Bruyn, P.L.; Modi, H.J. Particle Size and Flotation Rate of Quartz. Am. Inst. Min. Metall. Pet. Eng. 1956, 205, 415–419. [Google Scholar]
- Holland-Batt, A.B. Efficiency in batch separations. Inst. Mater. Miner. Min. 1971, 80, C12–C23. [Google Scholar]
- Sahoo, S.K.; Suresh, N.; Varma, A.K. Performance Evaluation of Basic Flotation Kinetic Models Using Advanced Statistical Techniques. Int. J. Coal Prep. Util. 2019, 39, 65–87. [Google Scholar] [CrossRef]
- Sokolovic, J.; Miskovic, S. The effect of particle size on coal flotation kinetics: A review. Physicochem. Probl. Miner. Process. 2018, 54, 1172–1190. [Google Scholar]
- Vinnett, L.; Alvarez-Silva, M.; Jaques, A.; Hinojosa, F.; Yianatos, J. Batch flotation kinetics: Fractional calculus approach. Miner. Eng. 2015, 77, 167–171. [Google Scholar] [CrossRef]
- Tarasov, V.E. Fractional Dynamics: Applications of Fractional Calculus to Dynamics of Particles, Fields and Media; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2011. [Google Scholar]
- Alvarez-Silva, M.; Vinnett, L.; Langlois, R.; Waters, K.E. A comparison of the predictability of batch flotation kinetic models. Miner. Eng. 2016, 99, 142–150. [Google Scholar] [CrossRef]
- Zhang, X.; Han, Y.; Gao, P.; Li, Y.; Sun, Y. Effects of particle size and ferric hydroxo complex produced by different grinding media on the flotation kinetics of pyrite. Powder Technol. 2020, 360, 1028–1036. [Google Scholar] [CrossRef]
- Hassanzadeh, A.; Hoang, D.H.; Brockmann, M. Assessment of flotation kinetics modeling using information criteria; case studies of elevated-pyritic copper sulfide and high-grade carbonaceous sedimentary apatite ores. J. Dispers. Sci. Technol. 2020, 41, 1083–1094. [Google Scholar] [CrossRef]
- Liao, Y.; Cao, Y.; Liu, C.; Zhu, G. A Study of Kinetics on Oily-Bubble Flotation for a Low-Rank Coal. Int. J. Coal Prep. Util. 2016, 36, 151–162. [Google Scholar] [CrossRef]
- Bu, X.; Xie, G.; Peng, Y.; Chen, Y. Kinetic modeling and optimization of flotation process in a cyclonic microbubble flotation column using composite central design methodology. Int. J. Miner. Process. 2016, 157, 175–183. [Google Scholar] [CrossRef]
- Ma, G.; Xia, W.; Xie, G. Effect of particle shape on the flotation kinetics of fine coking coal. J. Clean. Prod. 2018, 195, 470–475. [Google Scholar] [CrossRef]
- Mao, Y.; Bu, X.; Peng, Y.; Tian, F.; Xie, G. Effects of simultaneous ultrasonic treatment on the separation selectivity and flotation kinetics of high-ash lignite. Fuel 2020, 259, 116270. [Google Scholar] [CrossRef]
- Chen, S.; Tao, X.; Wang, S.; Tang, L.; Liu, Q.; Li, L. Comparison of air and oily bubbles flotation kinetics of long-flame coal. Fuel 2019, 236, 636–642. [Google Scholar] [CrossRef]
- Yang, L.; Li, D.; Zhang, H.; Yan, X. Flotation kinetics of the removal of unburned carbon from coal fly ash. Energy Sources Part A Recovery Util. Environ. Eff. 2018, 40, 1781–1787. [Google Scholar] [CrossRef]
- Kazemi, F.; Bahrami, A.; Abdollahisharif, J. Determination of the difference in recovery and kinetics of various size fractions of gilsonite in rougher and cleaner flotation processes. Int. J. Min. Geo-Eng. 2019, 53, 25–31. [Google Scholar]
- Sahoo, S.K.; Suresh, N.; Varma, A.K. Determining the Best Particle Size-Class for Flotation of a High Ash Coal. Int. J. Coal Prep. Util. 2017, 1–11. [Google Scholar] [CrossRef]
- Ahmadi, M.; Gharabaghi, M.; Abdollahi, H. Effects of type and dosages of organic depressants on pyrite floatability in microflotation system. Adv. Powder Technol. 2018, 29, 3155–3162. [Google Scholar] [CrossRef]
- Gharai, M.; Venugopal, R. Modeling of flotation process—An overview of different approaches. Miner. Process. Extr. Metall. Rev. 2016, 37, 120–133. [Google Scholar] [CrossRef]
- Laskowski, J.S. Flotation machines. In Developments in Mineral Processing; Elsevier: Amsterdam, The Netherlands, 2001; Volume 14, pp. 225–262. [Google Scholar]
- Mendez, D.A.; Gálvez, E.D.; Cisternas, L.A. State of the art in the conceptual design of flotation circuits. Int. J. Miner. Process. 2009, 90, 1–15. [Google Scholar] [CrossRef]
- Polat, M.; Chander, S. First-order flotation kinetics models and methods for estimation of the true distribution of flotation rate constants. Int. J. Miner. Process. 2000, 58, 145–166. [Google Scholar] [CrossRef]
- Hernáinz, F.; Calero, M. Froth flotation: Kinetic models based on chemical analogy. Chem. Eng. Process. Process Intensif. 2001, 40, 269–275. [Google Scholar] [CrossRef]
- Mehrotra, S.P.; Kapur, P.C. The effects of aeration rate, particle size and pulp density on the flotation rate distributions. Powder Technol. 1974, 9, 213–219. [Google Scholar] [CrossRef]
- Pascual, R.L.; Whiten, W.J. The determination of floatability distribution from laboratory batch cell tests. Miner. Eng. 2015, 83, 1–12. [Google Scholar] [CrossRef]
- Vinnett, L.; Navarra, A.; Waters, K.E. Comparison of different methodologies to estimate the flotation rate distribution. Miner. Eng. 2019, 130, 67–75. [Google Scholar] [CrossRef]
- Valsa, J.; Brančik, L. Approximate formulae for numerical inversion of Laplace transforms. Int. J. Numer. Model. Electron. Netw. Devices Fields 1998, 11, 153–166. [Google Scholar] [CrossRef]
- Vinnett, L.; Marion, C.; Grammatikopoulos, T.; Waters, K.E. Analysis of flotation rate distributions to assess erratic performances from size-by-size kinetic tests. Miner. Eng. 2020, 149, 106229. [Google Scholar] [CrossRef]
- Boudreau, B.P.; Ruddick, B.R. On a reactive continuum representation of organic matter diagenesis. Am. J. Sci. 1991, 291, 507–538. [Google Scholar] [CrossRef]
- Astarita, G. Lumping nonlinear kinetics: Apparent overall order of reaction. AICHE J. 1989, 35, 529–532. [Google Scholar] [CrossRef]
- Okino, M.S.; Mavrovouniotis, M.L. Simplification of mathematical models of chemical reaction systems. Chem. Rev. 1998, 98, 391–408. [Google Scholar] [CrossRef]
- Aris, R. Reactions in continuous mixtures. AICHE J. 1989, 35, 539–548. [Google Scholar] [CrossRef]
- Hyacinthe, C.; Van Cappellen, P. An authigenic iron phosphate phase in estuarine sediments: Composition, formation and chemical reactivity. Mar. Chem. 2004, 91, 227–251. [Google Scholar] [CrossRef]
- Debnath, L.; Bhatta, D. Integral Transforms and Their Applications, 2nd ed.; Chapman & Hall/CRC: Boca Raton, FL, USA, 2007. [Google Scholar]
- Andrews, R.N.; Narayanan, S.; Zhang, F.; Kuzmenko, I.; Ilavsky, J. Inverse transformation: Unleashing spatially heterogeneous dynamics with an alternative approach to XPCS data analysis. J. Appl. Crystallogr. 2018, 51, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Pollard, H. The representation of exp(-xλ) as a Laplace integral. Bull. Am. Math. Soc. 1946, 52, 908–910. [Google Scholar] [CrossRef] [Green Version]
- Dishon, M.; Bendler, J.T.; Weiss, G.H. Tables of the Inverse Laplace Transform of the Function exp(-sb). J. Res. Natl. Inst. Stand. Technol. 1990, 95, 433–467. [Google Scholar] [CrossRef]
- Penson, K.; Górska, K. Exact and explicit probability densities for one-sided Lévy stable distributions. Phys. Rev. Lett. 2010, 105, 210604. [Google Scholar] [CrossRef] [Green Version]
- Johnston, D. Stretched exponential relaxation arising from a continuous sum of exponential decays. Phys. Rev. B 2006, 74, 184430. [Google Scholar] [CrossRef] [Green Version]
- Gorenflo, R.; Mainardi, F. Fractional Calculus. In Fractals and Fractional Calculus in Continuum Mechanics; Carpinteri, A., Mainardi, F., Eds.; Springer: Vienna, Austria, 1997; pp. 223–276. [Google Scholar]








| Model | f(k) | R(t) |
|---|---|---|
| Single Rate Constant | ||
| Rectangular | ||
| Exponential | ||
| Gamma |
| 2nd-Order Model with Single Rate Constant k2 | First-Order Model with Exponential f(k) |
|---|---|
| nth-Order Model with Single Rate Constant kn | First-Order Model with Gamma f(k) |
|---|---|
| Second-Order Model with Rectangular f(k) [0-kmax] | Distributed First-Order Model |
|---|---|
| Rosin-Rammler Model | Distributed First-Order Model |
|---|---|
| Fractional Kinetics | Distributed First-Order Model |
|---|---|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vinnett, L.; Waters, K.E. Representation of Kinetics Models in Batch Flotation as Distributed First-Order Reactions. Minerals 2020, 10, 913. https://doi.org/10.3390/min10100913
Vinnett L, Waters KE. Representation of Kinetics Models in Batch Flotation as Distributed First-Order Reactions. Minerals. 2020; 10(10):913. https://doi.org/10.3390/min10100913
Chicago/Turabian StyleVinnett, Luis, and Kristian E. Waters. 2020. "Representation of Kinetics Models in Batch Flotation as Distributed First-Order Reactions" Minerals 10, no. 10: 913. https://doi.org/10.3390/min10100913