Higher Sensitivity of Soil Microbial Network Than Community Structure under Acid Rain
Abstract
:1. Introduction
2. Materials and Methods
2.1. Collection of Soil Cores
2.2. Experimental Design
2.3. Measurement of N2O Flux
2.4. Soil Sampling and Soil Physicochemical Properties Analysis
2.5. DNA Extraction, PCR Amplification, and Functional Genes Sequence Analysis
2.6. Network Inference, Construction, and Visualization
2.7. Statistical Analysis
3. Results
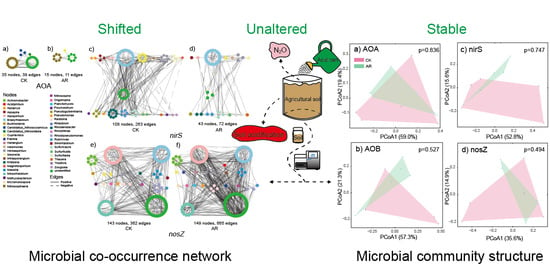
3.1. The AR Effects on Microbial Co-occurrence Networks
3.2. The AR Effects on Microbial Diversity, Community Structure, and N2O Emissions
4. Discussion
4.1. Impact of Acid Rain on Microbial Co-occurrence Patterns of AOA, nirS-, and nosZ-carrying Denitrifiers
4.2. Impact of Acid Rain on Microbial Diversity, Community Structure, and N2O Emissions
4.3. Idiosyncratic Responses of Microbial Co-occurrence Network, Community Structure, and Function to Acid Rain
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, X.; Zhang, B.; Zhao, W.; Wang, L.; Xie, D.; Huo, W.; Wu, Y.; Zhang, J. Comparative effects of sulfuric and nitric acid rain on litter decomposition and soil microbial community in subtropical plantation of Yangtze River Delta region. Sci. Total Environ. 2017, 601–602, 669–678. [Google Scholar] [CrossRef]
- Liu, Z.; Yang, J.; Zhang, J.; Xiang, H.; Wei, H. A bibliometric analysis of research on acid rain. Sustainability 2019, 11, 3077. [Google Scholar] [CrossRef] [Green Version]
- Singh, A.; Agrawal, M. Acid rain and its ecological consequences. J. Environ. Biol. 2008, 29, 15–24. [Google Scholar]
- Wei, H.; Ma, R.; Zhang, J.; Zhou, L.; Liu, Z.; Fan, Z.; Yang, J.; Shan, X.; Xiang, H. Quality dependence of litter decomposition and its carbon, nitrogen and phosphorus release under simulated acid rain treatments. Environ. Sci. Pollut. R. 2020, 27, 19858–19868. [Google Scholar] [CrossRef]
- Xu, H.; Zhang, J.; Ouyang, Y.; Lin, L.; Quan, G.; Zhao, B.; Yu, J. Effects of simulated acid rain on microbial characteristics in a lateritic red soil. Environ. Sci. Pollut. R. 2015, 22, 18260–18266. [Google Scholar] [CrossRef]
- The European Monitoring and Evaluation Programme (EMEP) Officially Reported Emission Trends. Available online: http://www.emep.int/ (accessed on 21 January 2019).
- The, U.S. Environmental Protection Agency (EPA) Our Nation’s Air: Air Quality Improves as America Grows. Available online: https://gispub.epa.gov/air/trendsreport/2018 (accessed on 21 January 2019).
- Balasubramanian, R.; Victor, T.; Begum, R. Impact of biomass burning on rainwater acidity and composition in Singapore. J. Geophys. Res. Atmos. 1999, 104, 26881–26890. [Google Scholar] [CrossRef]
- Kita, I.; Sato, T.; Kase, Y.; Mitropoulos, P. Neutral rains at Athens, Greece: A natural safeguard against acidification of rains. Sci. Total Environ. 2004, 327, 285–294. [Google Scholar] [CrossRef]
- Sant’Anna-Santos, B.F.; Da Silva, L.C.; Azevedo, A.A.; de Araujo, J.M.; Alves, E.F.; Da Silva, E.A.M.; Aguiar, R. Effects of simulated acid rain on the foliar micromorphology and anatomy of tree tropical species. Environ. Exp. Bot. 2006, 58, 158–168. [Google Scholar] [CrossRef]
- Wei, H.; Ma, R.; Zhang, J.; Saleem, M.; Liu, Z.; Shan, X.; Yang, J.; Xiang, H. Crop-litter type determines the structure and function of litter-decomposing microbial communities under acid rain conditions. Sci. Total Environ. 2020, 713, 136600. [Google Scholar] [CrossRef]
- Ministry of Ecological Environment of the People’s Republic of China. Available online: http://www.mee.gov.cn/ (accessed on 6 June 2019).
- Network Center for EANET EANET Data on the Acid Deposition in the East Asian Region. Available online: https://monitoring.eanet.asia/document/public/index (accessed on 26 September 2019).
- Harper, C.W.; Blair, J.M.; Fay, P.A.; Knapp, A.K.; Carlisle, J.D. Increased rainfall variability and reduced rainfall amount decreases soil CO2 flux in a grassland ecosystem. Glob. Chang. Biol. 2005, 11, 322–334. [Google Scholar] [CrossRef]
- Saleem, M. Microbiome ecosystem ecology: Unseen majority in an anthropogenic ecosystem. In Microbiome Community Ecology: Fundamentals and Applications; Saleem, M., Ed.; Springer International Publishing: Cham, Switzerland, 2015; pp. 1–11. [Google Scholar]
- Cai, Z.; Zhang, J.; Zhu, T.; Cheng, Y. Stimulation of NO and N2O emissions from soils by SO2 deposition. Glob. Chang. Biol. 2012, 18, 2280–2291. [Google Scholar] [CrossRef]
- Fan, J.; Xu, Y.; Chen, Z.; Xiao, J.; Liu, D.; Luo, J.; Bolan, N.; Ding, W. Sulfur deposition suppressed nitrogen-induced soil N2O emission from a subtropical forestland in southeastern China. Agric. Forest Meteorol. 2017, 233, 163–170. [Google Scholar] [CrossRef]
- Wang, C.; Wang, W.; Sardans, J.; An, W.; Zeng, C.; Abid, A.A.; Peñuelas, J. Effect of simulated acid rain on CO2, CH4 and N2O fluxes and rice productivity in a subtropical Chinese paddy field. Environ. Pollut. 2018, 243, 1196–1205. [Google Scholar] [CrossRef]
- Nicol, G.W.; Leininger, S.; Schleper, C.; Prosser, J.I. The influence of soil pH on the diversity, abundance and transcriptional activity of ammonia oxidizing archaea and bacteria. Environ. Microbiol. 2008, 10, 2966–2978. [Google Scholar] [CrossRef]
- Liu, B.; Morkved, P.T.; Frostegard, A.; Bakken, L.R. Denitrification gene pools, transcription and kinetics of NO, N2O and N2 production as affected by soil pH. FEMS Microbiol. Ecol. 2010, 72, 407–417. [Google Scholar]
- Levy-Booth, D.J.; Prescott, C.E.; Grayston, S.J. Microbial functional genes involved in nitrogen fixation, nitrification and denitrification in forest ecosystems. Soil Biol. Biochem. 2014, 75, 11–25. [Google Scholar] [CrossRef]
- Attard, E.; Recous, S.; Chabbi, A.; De Berranger, C.; Guillaumaud, N.; Labreuche, J.; Philippot, L.; Schmid, B.; Le Roux, X. Soil environmental conditions rather than denitrifier abundance and diversity drive potential denitrification after changes in land uses. Glob. Chang. Biol. 2011, 17, 1975–1989. [Google Scholar] [CrossRef]
- Liu, X.; Zhao, W.; Meng, M.; Fu, Z.; Xu, L.; Zha, Y.; Yue, J.; Zhang, S.; Zhang, J. Comparative effects of simulated acid rain of different ratios of SO42− to NO3− on fine root in subtropical plantation of China. Sci. Total Environ. 2018, 618, 336–346. [Google Scholar] [CrossRef]
- Liu, X.; Zhou, J.; Li, W.; Xu, J.; Brookes, P.C. The combined effects of urea application and simulated acid rain on soil acidification and microbial community structure. Environ. Sci. Pollut. Res. 2014, 21, 6623–6631. [Google Scholar] [CrossRef]
- Jalali, M.; Naderi, E. The impact of acid rain on phosphorus leaching from a sandy loam calcareous soil of western Iran. Environ. Earth Sci. 2012, 66, 311–317. [Google Scholar] [CrossRef]
- Wang, L.; Chen, Z.; Shang, H.; Wang, J.; Zhang, P. Impact of simulated acid rain on soil microbial community function in Masson pine seedlings. Electron. J. Biotechn. 2014, 17, 199–203. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Wang, Y.; Wang, Y.; Wang, B. Effects of simulated acid rain on soil respiration and its component in a mixed coniferous-broadleaved forest of the three gorges reservoir area in Southwest China. For. Ecosyst. 2019, 6, 32. [Google Scholar] [CrossRef] [Green Version]
- Saleem, M.; Moe, L.A. Multitrophic microbial interactions for eco- and agro-biotechnological processes: Theory and practice. Trends Biotechnol. 2014, 32, 529–537. [Google Scholar] [CrossRef] [PubMed]
- Faust, K.; Sathirapongsasuti, J.F.; Izard, J.; Segata, N.; Gevers, D.; Raes, J.; Huttenhower, C. Microbial co-occurrence relationships in the human microbiome. PLoS Comput. Biol. 2012, 8, e1002606. [Google Scholar] [CrossRef] [PubMed]
- Mamet, S.D.; Redlick, E.; Brabant, M.; Lamb, E.G.; Helgason, B.L.; Stanley, K.; Siciliano, S.D. Structural equation modeling of a winnowed soil microbiome identifies how invasive plants re-structure microbial networks. ISME J. 2019, 13, 1988–1996. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Zhang, J.; Liu, Y.; Shi, P.; Wei, G. Co-occurrence patterns of soybean rhizosphere microbiome at a continental scale. Soil Biol. Biochem. 2018, 118, 178–186. [Google Scholar] [CrossRef]
- Saleem, M.; Hu, J.; Jousset, A. More than the sum of its parts: Microbiome biodiversity as a driver of plant growth and soil health. Annu. Rev. Ecol. Evol. Syst. 2019, 50, 145–168. [Google Scholar] [CrossRef]
- Chow, C.T.; Kim, D.Y.; Sachdeva, R.; Caron, D.A.; Fuhrman, J.A. Top-down controls on bacterial community structure: Microbial network analysis of bacteria, T4-like viruses and protists. ISME J. 2014, 8, 816–829. [Google Scholar] [CrossRef] [Green Version]
- Lima-Mendez, G.; Faust, K.; Henry, N.; Decelle, J.; Colin, S.; Carcillo, F.; Chaffron, S.; Ignacio-Espinosa, J.C.; Roux, S.; Vincent, F.; et al. Determinants of community structure in the global plankton interactome. Science 2015, 348, 1262073. [Google Scholar] [CrossRef] [Green Version]
- Steele, J.A.; Countway, P.D.; Xia, L.; Vigil, P.D.; Beman, J.M.; Kim, D.Y.; Chow, C.T.; Sachdeva, R.; Jones, A.C.; Schwalbach, M.S.; et al. Marine bacterial, archaeal and protistan association networks reveal ecological linkages. ISME J. 2011, 5, 1414–1425. [Google Scholar] [CrossRef]
- Wei, H.; Zhang, K.; Zhang, J.; Li, D.; Zhang, Y.; Xiang, H. Grass cultivation alters soil organic carbon fractions in a subtropical orchard of southern China. Soil Tillage Res. 2018, 181, 110–116. [Google Scholar] [CrossRef]
- Liu, Z.; Li, D.; Zhang, J.; Saleem, M.; Zhang, Y.; Ma, R.; He, Y.; Yang, J.; Xiang, H.; Wei, H. Effect of simulated acid rain on soil CO2, CH4 and N2O emissions and microbial communities in an agricultural soil. Geoderma 2020, 366, 114222. [Google Scholar] [CrossRef]
- Ministry of Ecology and Environment of the People’s Republic of China Bulletin on the State of China’s Ecological Environment in 2011. Available online: http://www.mee.gov.cn/ (accessed on 6 June 2019).
- Guangdong Meteorological Bureau. Available online: http://gd.cma.gov.cn/ (accessed on 6 June 2019).
- Department of Ecological Environment of Guangdong Province. Available online: https://www.gdep.gov.cn/ (accessed on 6 June 2019).
- Shi, Y.; Liu, X.; Zhang, Q.; Gao, P.; Ren, J. Biochar and organic fertilizer changed the ammonia-oxidizing bacteria and archaea community structure of saline–alkali soil in the North China Plain. J. Soil. Sediment. 2019, 20, 12–23. [Google Scholar] [CrossRef]
- Faust, K.; Raes, J. CoNet app: Inference of biological association networks using Cytoscape. F1000Research 2016, 5, 1519. [Google Scholar] [CrossRef]
- Navarrete, A.A.; Tsai, S.M.; Mendes, L.W.; Faust, K.; de Hollander, M.; Cassman, N.A.; Raes, J.; van Veen, J.A.; Kuramae, E.E. Soil microbiome responses to the short-term effects of Amazonian deforestation. Mol. Ecol. 2015, 24, 2433–2448. [Google Scholar] [CrossRef]
- Berry, D.; Widder, S. Deciphering microbial interactions and detecting keystone species with co-occurrence networks. Front. Microbiol. 2014, 5, 219. [Google Scholar]
- R Core Team R: A language and environment for statistical computing. R Foundation for Statistical Computing. Available online: https://www.R-project.org/ (accessed on 4 June 2019).
- Chen, J.; Xiao, G.; Kuzyakov, Y.; Jenerette, G.D.; Ma, Y.; Liu, W.; Wang, Z.; Shen, W. Soil nitrogen transformation responses to seasonal precipitation changes are regulated by changes in functional microbial abundance in a subtropical forest. Biogeosciences 2017, 14, 2513–2525. [Google Scholar] [CrossRef] [Green Version]
- Banerjee, S.; Walder, F.; Büchi, L.; Meyer, M.; Held, A.Y.; Gattinger, A.; Keller, T.; Charles, R.; van der Heijden, M.G.A. Agricultural intensification reduces microbial network complexity and the abundance of keystone taxa in roots. ISME J. 2019, 13, 1722–1736. [Google Scholar]
- Ma, B.; Wang, H.; Dsouza, M.; Lou, J.; He, Y.; Dai, Z.; Brookes, P.C.; Xu, J.; Gilbert, J.A. Geographic patterns of co-occurrence network topological features for soil microbiota at continental scale in eastern China. ISME J. 2016, 10, 1891–1901. [Google Scholar] [CrossRef]
- Shi, S.; Nuccio, E.E.; Shi, Z.J.; He, Z.; Zhou, J.; Firestone, M.K. The interconnected rhizosphere: High network complexity dominates rhizosphere assemblages. Ecol. Lett. 2016, 19, 926–936. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Chen, Z.; He, J.; Wang, Q.; Shen, C.; Ge, Y. Ectomycorrhizal fungi inoculation alleviates simulated acid rain effects on soil ammonia oxidizers and denitrifiers in Masson pine forest. Environ. Microbiol. 2019, 21, 299–313. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Deng, Y.; Luo, F.; He, Z.; Yang, Y. Phylogenetic molecular ecological network of soil microbial communities in response to elevated CO2. Mbio 2011, 2. [Google Scholar] [CrossRef] [Green Version]
- Berendsen, R.L.; Pieterse, C.M.J.; Bakker, P.A.H.M. The rhizosphere microbiome and plant health. Trends Plant Sci. 2012, 17, 478–486. [Google Scholar] [CrossRef]
- Jones, R.T.; Robeson, M.S.; Lauber, C.L.; Hamady, M.; Knight, R.; Fierer, N. A comprehensive survey of soil acidobacterial diversity using pyrosequencing and clone library analyses. ISME J. 2009, 3, 442–453. [Google Scholar] [CrossRef]
- Liang, Y.; Zhao, H.; Deng, Y.; Zhou, J.; Li, G.; Sun, B. Long-Term oil contamination alters the molecular ecological networks of soil microbial functional genes. Front. Microbiol. 2016, 7, 60. [Google Scholar] [CrossRef] [Green Version]
- Nielsen, S.; Minchin, T.; Kimber, S.; van Zwieten, L.; Gilbert, J.; Munroe, P.; Joseph, S.; Thomas, T. Comparative analysis of the microbial communities in agricultural soil amended with enhanced biochars or traditional fertilisers. Agric. Ecosyst. Environ. 2014, 191, 73–82. [Google Scholar] [CrossRef]
- Chapin, F.R.; Zavaleta, E.S.; Eviner, V.T.; Naylor, R.L.; Vitousek, P.M.; Reynolds, H.L.; Hooper, D.U.; Lavorel, S.; Sala, O.E.; Hobbie, S.E.; et al. Consequences of changing biodiversity. Nature 2000, 405, 234–242. [Google Scholar] [CrossRef]
- Faust, K.; Raes, J. Microbial interactions: From networks to models. Nat. Rev. Microbiol. 2012, 10, 538–550. [Google Scholar] [CrossRef]
- Liu, W.; Graham, E.B.; Dong, Y.; Zhong, L.; Zhang, J.; Qiu, C.; Chen, R.; Lin, X.; Feng, Y. Balanced stochastic versus deterministic assembly processes benefit diverse yet uneven ecosystem functions in representative agroecosystems. Environ. Microbiol. 2020. [Google Scholar] [CrossRef]
- de Vries, F.T.; Griffiths, R.I.; Bailey, M.; Craig, H.; Girlanda, M.; Gweon, H.S.; Hallin, S.; Kaisermann, A.; Keith, A.M.; Kretzschmar, M.; et al. Soil bacterial networks are less stable under drought than fungal networks. Nat. Commun. 2018, 9, 3033. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Zhang, L.; Shen, J.; Wei, W.; He, J. Abundance and community structure of ammonia-oxidizing archaea and bacteria in an acid paddy soil. Biol. Fert. Soils 2011, 47, 323–331. [Google Scholar] [CrossRef]
- He, J.; Shen, J.; Zhang, L.; Zhu, Y.; Zheng, Y.; Xu, M.; Di, H. Quantitative analyses of the abundance and composition of ammonia-oxidizing bacteria and ammonia-oxidizing archaea of a Chinese upland red soil under long-term fertilization practices. Environ. Microbiol. 2007, 9, 2364–2374. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Yu, G.; Zhang, X.; Wang, Q.; Tian, D.; Tian, J.; Niu, S.; Ge, J. Environmental variables better explain changes in potential nitrification and denitrification activities than microbial properties in fertilized forest soils. Sci. Total Environ. 2019, 647, 653–662. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Xing, W.; Lan, Z.; Saleem, M.; Wu, Y.; Hu, S.; Bai, Y. Direct and indirect effects of nitrogen enrichment on soil organisms and carbon and nitrogen mineralization in a semi-arid grassland. Funct. Ecol. 2019, 33, 175–187. [Google Scholar] [CrossRef] [Green Version]
- Chen, D.; Saleem, M.; Cheng, J.; Mi, J.; Chu, P.; Tuvshintogtokh, I.; Hu, S.; Bai, Y. Effects of aridity on soil microbial communities and functions across soil depths on the Mongolian Plateau. Funct. Ecol. 2019, 33, 1561–1571. [Google Scholar] [CrossRef]
- Ling, N.; Zhu, C.; Xue, C.; Chen, H.; Duan, Y.; Peng, C.; Guo, S.; Shen, Q. Insight into how organic amendments can shape the soil microbiome in long-term field experiments as revealed by network analysis. Soil Biol. Biochem. 2016, 99, 137–149. [Google Scholar] [CrossRef]
- Vanhala, P.; Fritze, H.; Neuvonen, S. Prolonged simulated acid rain treatment in the subarctic: Effect on the soil respiration rate and microbial biomass. Biol. Fert. Soils 1996, 23, 7–14. [Google Scholar] [CrossRef]
- Wang, C.; Guo, P.; Han, G.; Feng, X.; Zhang, P.; Tian, X. Effect of simulated acid rain on the litter decomposition of Quercus acutissima and Pinus massoniana in forest soil microcosms and the relationship with soil enzyme activities. Sci. Total Environ. 2010, 408, 2706–2713. [Google Scholar] [CrossRef]
- Tang, Y.; Zhang, X.; Li, D.; Wang, H.; Chen, F.; Fu, X.; Fang, X.; Sun, X.; Yu, G. Impacts of nitrogen and phosphorus additions on the abundance and community structure of ammonia oxidizers and denitrifying bacteria in Chinese fir plantations. Soil Biol. Biochem. 2016, 103, 284–293. [Google Scholar] [CrossRef]
- Malik, A.A.; Martiny, J.B.H.; Brodie, E.L.; Martiny, A.C.; Treseder, K.K.; Allison, S.D. Defining trait-based microbial strategies with consequences for soil carbon cycling under climate change. ISME J. 2019, 14, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Petersen, D.G.; Blazewicz, S.J.; Firestone, M.; Herman, D.J.; Turetsky, M.; Waldrop, M. Abundance of microbial genes associated with nitrogen cycling as indices of biogeochemical process rates across a vegetation gradient in Alaska. Environ. Microbiol. 2012, 14, 993–1008. [Google Scholar] [CrossRef]
- Heinen, M. Simplified denitrification models: Overview and properties. Geoderma 2006, 133, 444–463. [Google Scholar] [CrossRef]
- Lozanovska, I.; Kuzyakov, Y.; Krohn, J.; Parvin, S.; Dorodnikov, M. Effects of nitrate and sulfate on greenhouse gas emission potentials from microform-derived peats of a boreal peatland: A 13 C tracer study. Soil Biol. Biochem. 2016, 100, 182–191. [Google Scholar] [CrossRef]
- Pu, G.; Saffigna, P.G.; Xu, Z. Denitrification, leaching and immobilisation of 15 N-labelled nitrate in winter under windrowed harvesting residues in hoop pine plantations of 1–3 years old in subtropical Australia. Forest Ecol. Manag. 2001, 152, 183–194. [Google Scholar] [CrossRef]
- Šimek, M.; Cooper, J.E.; Picek, T.; Šantrůčková, H. Denitrification in arable soils in relation to their physico-chemical properties and fertilization practice. Soil Biol. Biochem. 2000, 32, 101–110. [Google Scholar] [CrossRef]
- Sitaula, B.K.; Bakken, L.R.; Abrahamsen, G. N-fertilization and soil acidification effects on N2O and CO2 emission from temperate pine forest soil. Soil Biol. Biochem. 1995, 27, 1401–1408. [Google Scholar]
- Morales, S.E.; Cosart, T.; Holben, W.E. Bacterial gene abundances as indicators of greenhouse gas emission in soils. ISME J. 2010, 4, 799–808. [Google Scholar] [CrossRef]
- Rasche, F.; Knapp, D.; Kaiser, C.; Koranda, M.; Kitzler, B.; Zechmeister-Boltenstern, S.; Richter, A.; Sessitsch, A. Seasonality and resource availability control bacterial and archaeal communities in soils of a temperate beech forest. ISME J. 2011, 5, 389–402. [Google Scholar] [CrossRef] [Green Version]
- Allison, S.D.; Martiny, J.B. Colloquium paper: Resistance, resilience, and redundancy in microbial communities. Proc. Natl. Acad. Sci. USA 2008, 105 (Suppl. 1), 11512–11519. [Google Scholar] [CrossRef] [Green Version]
- Malik, A.A.; Puissant, J.; Goodall, T.; Allison, S.D.; Griffiths, R.I. Soil microbial communities with greater investment in resource acquisition have lower growth yield. Soil Biol. Biochem. 2019, 132, 36–39. [Google Scholar] [CrossRef] [Green Version]





| Microbial Community | Topological Features | |||||||
|---|---|---|---|---|---|---|---|---|
| Treatment | Nodes a | Edges b | Clustering Coefficient c | Network Diameter d | Shortest Paths e | Avg. Number of Neighbors f | Graph Density g | |
| AOA | CK | 35 | 38 | 0.424 | 2 | 88 | 2.171 | 0.064 |
| AR | 15 | 11 | 0.400 | 2 | 24 | 1.467 | 0.105 | |
| nirS | CK | 108 | 263 | 0.723 | 10 | 5850 | 4.870 | 0.046 |
| AR | 43 | 72 | 0.756 | 2 | 164 | 3.349 | 0.080 | |
| nosZ | CK | 143 | 362 | 0.773 | 7 | 8756 | 5.063 | 0.036 |
| AR | 149 | 665 | 0.701 | 9 | 16248 | 8.926 | 0.060 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Wei, H.; Zhang, J.; Saleem, M.; He, Y.; Zhong, J.; Ma, R. Higher Sensitivity of Soil Microbial Network Than Community Structure under Acid Rain. Microorganisms 2021, 9, 118. https://doi.org/10.3390/microorganisms9010118
Liu Z, Wei H, Zhang J, Saleem M, He Y, Zhong J, Ma R. Higher Sensitivity of Soil Microbial Network Than Community Structure under Acid Rain. Microorganisms. 2021; 9(1):118. https://doi.org/10.3390/microorganisms9010118
Chicago/Turabian StyleLiu, Ziqiang, Hui Wei, Jiaen Zhang, Muhammad Saleem, Yanan He, Jiawen Zhong, and Rui Ma. 2021. "Higher Sensitivity of Soil Microbial Network Than Community Structure under Acid Rain" Microorganisms 9, no. 1: 118. https://doi.org/10.3390/microorganisms9010118