A Review of the Functional Annotations of Important Genes in the AHPND-Causing pVA1 Plasmid
Abstract
:1. Introduction
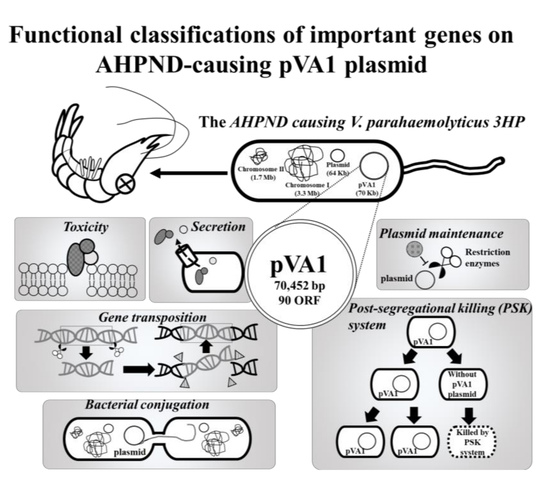
2. The AHPND-Causing Plasmid pVA1
2.1. PirAvp (ORF 51) and PirBvp (ORF50)
2.2. Transposases (ORF15, ORF48, ORF55, ORF57 and ORF68)
2.3. Conjugation Factors (ORF10, ORF11, ORF75, ORF76, ORF78, ORF79, ORF81, ORF82, ORF83, and ORF84)
2.4. Antirestriction Proteins (ORF32 and ORF35)
2.5. Secretion System (ORF3, 86, 89, and 90)
2.6. pndA (ORF7)
2.7. DNA Methyltransferase (ORF47 and ORF56)
3. Conclusion Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shinn, A.P.; Pratoomyot, J.; Griffiths, D.; Trong, T.Q.; Vu, N.T.; Jiravanichpaisal, P.; Briggs, M. Asian shrimp production and economic costs of disease. Asian Fish. Sci. 2018, 31S, 29–58. [Google Scholar]
- Eshik, M.M.E.; Abedin, M.M.; Punom, N.J.; Begum, M.K.; Rahman, M.S. Molecular identification of AHPND positive Vibrio parahaemolyticus causing an outbreak in south-west shrimp farming regions of Bangladesh. J. Bangladesh Acad. Sci. 2017, 41, 127–135. [Google Scholar] [CrossRef] [Green Version]
- Dhar, A.K.; Piamsomboon, P.; Caro, L.F.A.; Kanrar, S.; Adami, R.J.; Juan, Y.S. First report of acute hepatopancreatic necrosis disease (AHPND) occurring in the USA. Dis. Aquat. Org. 2019, 132, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Tran, L.; Nunan, L.; Redman, R.; Mohney, L.; Pantoja, C.; Fitzsimmons, K.; Lightner, D.V. Determination of the infectious nature of the agent of acute hepatopancreatic necrosis syndrome affecting penaeid shrimp. Dis. Aquat. Org. 2013, 105, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.T.; Chen, I.T.; Yang, Y.T.; Ko, T.P.; Huang, Y.T.; Huang, J.Y.; Huang, M.F.; Lin, S.J.; Chen, C.Y.; Lin, S.S.; et al. The opportunistic marine pathogen Vibrio parahaemolyticus becomes virulent by acquiring a plasmid that expresses a deadly toxin. Proc. Natl. Acad. Sci. USA 2015, 112, 10798–10803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lai, H.C.; Ng, T.H.; Ando, M.; Lee, C.T.; Chen, I.T.; Chuang, J.C.; Mavichak, R.; Chang, S.H.; Yeh, M.D.; Chiang, Y.A.; et al. Pathogenesis of acute hepatopancreatic necrosis disease (AHPND) in shrimp. Fish. Shellfish Immunol. 2015, 47, 1006–1014. [Google Scholar] [CrossRef] [PubMed]
- Tinwongger, S.; Nochiri, Y.; Thawonsuwan, J.; Nozaki, R.; Kondo, H.; Awasthi, S.P.; Hinenoya, A.; Yamasaki, S.; Hirono, I. Virulence of acute hepatopancreatic necrosis disease PirAB-like relies on secreted proteins not on gene copy number. J. Appl. Microbiol. 2016, 121, 1755–1765. [Google Scholar] [CrossRef]
- Theethakaew, C.; Nakamura, S.; Motooka, D.; Matsuda, S.; Kodama, T.; Chonsin, K.; Suthienkul, O.; Iida, T. Plasmid dynamics in Vibrio parahaemolyticus strains related to shrimp Acute Hepatopancreatic Necrosis Syndrome (AHPNS). Infect. Genet. Evol. 2017, 51, 211–218. [Google Scholar] [CrossRef]
- Lin, S.J.; Hsu, K.C.; Wang, H.C. Structural insights into the cytotoxic mechanism of Vibrio parahaemolyticus PirAvp and PirBvp toxins. Mar. Drugs. 2017, 15, 373. [Google Scholar] [CrossRef] [Green Version]
- Kumar, R.; Ng, T.H.; Wang, H.C. Acute hepatopancreatic necrosis disease in penaeid shrimp. Rev. Aquacult. 2020. [Google Scholar] [CrossRef] [Green Version]
- Letchumanan, V.; Chan, K.G.; Lee, L.H. Vibrio parahaemolyticus: A review on the pathogenesis, prevalence, and advance molecular identification techniques. Front. Microbiol. 2014, 5, 705. [Google Scholar] [CrossRef] [Green Version]
- Lightner, D.V.; Redman, R.M.; Pantoja, C.R.; Noble, B.L.; Tran, L. Early mortality syndrome affects shrimp in Asia. Glob. Aquac. Advocate 2012, 15, 40. [Google Scholar]
- Lin, S.J.; Chen, Y.F.; Hsu, K.C.; Chen, Y.L.; Ko, T.P.; Lo, C.F.; Wang, H.C.; Wang, H.C. Structural insights to the heterotetrameric interaction between the Vibrio parahaemolyticus PirAvp and PirBvp toxins and activation of the Cry-like pore-forming domain. Toxins 2019, 11, 233. [Google Scholar] [CrossRef] [Green Version]
- Kumar, R.; Ng, T.H.; Chang, C.C.; Lin, S.S.; Lo, C.F.; Wang, H.C. Bile acid and bile acid transporters are involved pathogenesis of acute hepatopancreatic necrosis disease in white shrimp Litopenaeus vannamei. Cel. Microbiol. 2019, 22, e13127. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Gil, B.; Soto-Rodriguez, S.; Lozano, R.; Betancourt-Lozano, M. Draft genome sequence of Vibrio parahaemolyticus strain M0605, which causes severe mortalities of shrimps in Mexico. Genome Announc. 2014, 2, e00055-14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.T.; Chen, I.T.; Lee, C.T.; Chen, C.Y.; Lin, S.S.; Hor, L.I.; Tseng, T.C.; Huang, Y.T.; Sritunyalucksana, K.; Thitamadee, S.; et al. Draft genome sequences of four strains of Vibrio parahaemolyticus, three of which cause early mortality syndrome/acute hepatopancreatic necrosis disease in shrimp in China and Thailand. Genome Announc. 2014, 2, e00816-14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kondo, H.; Tinwongger, S.; Proespraiwong, P.; Mavichak, R.; Unajak, S.; Nozaki, R.; Hirono, I. Draft genome sequences of six strains of Vibrio parahaemolyticus isolated from early mortality syndrome/acute hepatopancreatic necrosis disease shrimp in Thailand. Genome Announc. 2014, 2, e00221-14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gomez-Jimenez, S.; Noriega-Orozco, L.; Sotelo-Mundo, R.R.; Cantu-Robles, V.A.; Cobian-Guemes, A.G.; Cota-Verdugo, R.G.; Gamez-Alejo, L.A.; Del Pozo-Yauner, L.; Guevara-Hernandez, E.; Garcia-Orozco, K.D.; et al. High-quality draft genomes of two Vibrio parahaemolyticus strains aid in understanding acute hepatopancreatic necrosis disease of cultured shrimps in Mexico. Genome Announc. 2014, 2, e00800-14. [Google Scholar] [CrossRef] [Green Version]
- Han, J.; Tang, K.; Tran, L.; Lightner, D.V. Photorhabdus insect-related (Pir) toxin-like genes in a plasmid of Vibrio parahaemolyticus, the causative agent of acute hepatopancreatic necrosis disease (AHPND) of shrimp. Dis. Aquat. Org. 2015, 113, 33–40. [Google Scholar] [CrossRef] [Green Version]
- Dong, X.; Wang, H.; Xie, G.; Zou, P.; Guo, C.; Liang, Y.; Huang, J. An isolate of Vibrio campbellii carrying the pirVP gene causes acute hepatopancreatic necrosis disease. Emerg. Microbes Infect. 2017, 6, e2. [Google Scholar] [CrossRef] [Green Version]
- Kondo, H.; Van, P.T.; Dang, L.T.; Hirono, I. Draft genome sequence of non-Vibrio parahaemolyticus acute hepatopancreatic necrosis disease strain KC13.17.5, isolated from diseased shrimp in Vietnam. Genome Announc. 2015, 3, e00978-15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, L.; Xiao, J.; Xia, X.; Pan, Y.; Yan, S.; Wang, Y. Draft genome sequence of Vibrio owensii strain SH-14, which causes shrimp acute hepatopancreatic necrosis disease. Genome Announc. 2015, 3, e01395-15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Restrepo, L.; Bayot, B.; Arciniegas, S.; Bajaña, L.; Betancourt, I.; Panchana, F.; Reyes Muñoz, A. PirVPgenes causing AHPND identified in a new Vibrio species (Vibrio punensis) within the commensal Orientalis clade. Sci. Rep. 2018, 8, 13080. [Google Scholar] [CrossRef] [PubMed]
- Durán-Avelar, M.J.; Vázquez-Reyes, A.; González-Mercado, A.L.; Zambrano-Zaragoza, J.F.; Ayón-Pérez, M.F.; Agraz-Cibrián, J.M.; Gutiérrez-Franco, J.; Vibanco-Pérez, N. pirA- and pirB-like gene identification in Micrococcus luteus strains in Mexico. J. Fish. Dis. 2018, 41, 1667–1673. [Google Scholar] [CrossRef] [PubMed]
- Muthukrishnan, S.; Defoirdt, T.; Shariff, M.; Ina-Salwany, M.Y.; Yusoff, F.M.; Natrah, I. Horizontal gene transfer of the pirAB genes responsible for Acute Hepatopancreatic Necrosis Disease (AHPND) turns a non-Vibrio strain into an AHPND-positive pathogen. BioRxiv 2019. [Google Scholar] [CrossRef]
- Siguier, P.; Gourbeyre, E.; Chandler, M. Bacterial insertion sequences: Their genomic impact and diversity. FEMS Microbiol. Rev. 2014, 38, 865–891. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, S.; Wang, J.; Chitsaz, F.; Derbyshire, M.K.; Geer, R.C.; Gonzales, N.R.; Gwadz, M.; Hurwitz, D.I.; Marchler, G.H.; Song, J.S.; et al. CDD/SPARCLE: The conserved domain database in 2020. Nucleic Acids Res. 2020, 48, D265–D268. [Google Scholar] [CrossRef] [Green Version]
- Kingston, A.W.; Ponkratz, C.; Raleigh, E.A. Rpn (YhgA-Like) proteins of Escherichia coli K-12 and their contribution to RecA-independent horizontal transfer. J. Bacteriol. 2017, 199, e00787-16. [Google Scholar] [CrossRef] [Green Version]
- Green, E.R.; Mecsas, J. Bacterial secretion systems: An overview. Microbiol. Spectr. 2016, 4. [Google Scholar] [CrossRef] [Green Version]
- Haase, J.; Lurz, R.; Grahn, A.M.; Bamford, D.H.; Lanka, E. Bacterial conjugation mediated by plasmid RP4: RSF1010 mobilization, donor-specific phage propagation, and pilus production require the same Tra2 core components of a proposed DNA transport complex. J. Bacteriol. 1995, 177, 4779–4791. [Google Scholar] [CrossRef] [Green Version]
- Schröder, G.; Lanka, E. The mating pair formation system of conjugative plasmids-A versatile secretion machinery for transfer of proteins and DNA. Plasmid 2005, 54, 1–25. [Google Scholar] [CrossRef]
- Höppner, C.; Liu, Z.; Domke, N.; Binns, A.N.; Baron, C. VirB1 orthologs from Brucella suis and pKM101 complement defects of the lytic transglycosylase required for efficient type IV secretion from Agrobacterium tumefaciens. J. Bacteriol. 2004, 186, 1415–1422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belogurov, A.A.; Delver, E.P.; Rodzevich, O.V. Plasmid pKM101 encodes two nonhomologous antirestriction proteins (ArdA and ArdB) whose expression is controlled by homologous regulatory sequences. J. Bacteriol. 1993, 175, 4843–4850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belogurov, A.A.; Delver, E.P.; Agafonova, O.V.; Belogurova, N.G.; Lee, L.Y.; Kado, C.I. Antirestriction protein Ard (Type C) encoded by IncW plasmid pSa has a high similarity to the “protein transport” domain of TraC1 primase of promiscuous plasmid RP4. J. Mol. Biol. 2000, 296, 969–977. [Google Scholar] [CrossRef] [PubMed]
- Gerdes, K.; Nielsen, A.; Thorsted, P.; Wagner, E.G. Mechanism of killer gene activation. Antisense RNA-dependent RNase III cleavage ensures rapid turn-over of the stable hok, srnB and pndA effector messenger RNAs. J. Mol. Biol. 1992, 226, 637–649. [Google Scholar] [CrossRef]
- Julio, S.M.; Heithoff, D.M.; Provenzano, D.; Klose, K.E.; Sinsheimer, R.L.; Low, D.A.; Mahan, M.J. DNA adenine methylase is essential for viability and plays a role in the pathogenesis of Yersinia pseudotuberculosis and Vibrio cholerae. Infect. Immun. 2001, 69, 7610–7615. [Google Scholar] [CrossRef] [Green Version]
- McLuskey, K.; Harrison, J.A.; Schuttelkopf, A.W.; Boxer, D.H.; Hunter, W.N. Insight into the role of Escherichia coli MobB in molybdenum cofactor biosynthesis based on the high resolution crystal structure. J. Biol. Chem. 2003, 278, 23706–23713. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.M.; Wang, G.; Pertsinidis, A.; Marians, K.J. Topoisomerase III acts at the replication fork to remove precatenanes. J. Bacteriol. 2019, 2019 201, e00563-18. [Google Scholar] [CrossRef] [Green Version]
- Lecompte, O.; Ripp, R.; Thierry, J.C.; Moras, D.; Poch, O. Comparative analysis of ribosomal proteins in complete genomes: An example of reductive evolution at the domain scale. Nucleic Acids Res. 2002, 30, 5382–5390. [Google Scholar] [CrossRef]
- Tang, Q.; Liu, Y.P.; Shan, H.H.; Tian, L.F.; Zhang, J.Z.; Yan, X.X. ATP-dependent conformational change in ABC-ATPase RecF serves as a switch in DNA repair. Sci. Rep. 2018, 8, 2127. [Google Scholar] [CrossRef]
- Desai, B.V.; Morrison, D.A. An unstable competence-induced protein, CoiA, promotes processing of donor DNA after uptake during genetic transformation in Streptococcus pneumoniae. J. Bacteriol. 2006, 188, 5177–5186. [Google Scholar] [CrossRef] [Green Version]
- Surtees, J.A.; Funnell, B.E. Plasmid and chromosome traffic control: How ParA and ParB drive partition. Curr. Top. Dev. Biol. 2003, 56, 145–180. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, J.L. Bivalent sequential binding model of a Bacillus thuringiensis toxin to gypsy moth aminopeptidase N receptor. J. Biol. Chem. 2000, 275, 14423–14431. [Google Scholar] [CrossRef] [Green Version]
- Xu, C.; Wang, B.C.; Yu, Z.; Sun, M. Structural Insights into Bacillus thuringiensis Cry, Cyt and Parasporin Toxins. Toxins 2014, 6, 2732–2770. [Google Scholar] [CrossRef] [Green Version]
- Soberón, M.; Pardo, L.; Muñóz-Garay, C.; Sánchez, J.; Gómez, I.; Porta, H.; Bravo, A. Pore formation by Cry toxins. Adv. Exp. Med. Biol. 2010, 677, 127–142. [Google Scholar] [CrossRef] [PubMed]
- Almanza-Martínez, N.; Francisco Martínez Díaz, S.; Flores-Ramírez, G.; Zuñiga-Navarrete, F.; Gómez, I.; Cardona-Félix, C.S. An α-amylase-like protein interacts with PirB toxin from Vibrio parahaemolyticus in digestive tract tissue of white shrimp Litopenaeus vannamei. Aquac. Res. 2020, 1–5. [Google Scholar] [CrossRef]
- Victorio-De Los Santos, M.; Vibanco-Pérez, N.; Soto-Rodriguez, S.; Pereyra, A.; Zenteno, E.; Cano-Sánchez, P. The B subunit of PirABvp toxin secreted from Vibrio parahaemolyticus causing AHPND is an amino sugar specific lectin. Pathogens 2020, 9, 182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muñoz-López, M.; García-Pérez, J.L. DNA transposons: Nature and applications in genomics. Curr. Genom. 2010, 11, 115–128. [Google Scholar] [CrossRef] [Green Version]
- Xiao, J.; Liu, L.; Ke, Y.; Li, X.; Liu, Y.; Pan, Y.; Yan, S.; Wang, Y. Shrimp AHPND-causing plasmids encoding the PirAB toxins as mediated by pirAB-Tn903 are prevalent in various Vibrio species. Sci. Rep. 2017, 7, 42177. [Google Scholar] [CrossRef]
- Phiwsaiya, K.; Charoensapsri, W.; Taengphu, S.; Dong, H.T.; Sangsuriya, P.; Nguyen, G.T.T.; Pham, H.Q.; Amparyup, P.; Sritunyalucksana, K.; Taengchaiyaphum, S.; et al. A natural Vibrio parahaemolyticusΔpirAvp PirBvp+ mutant kills shrimp but produces no Pirvp toxins or AHPND lesions. Appl. Environ. Microbiol. 2017, 83, e00680-17. [Google Scholar] [CrossRef] [Green Version]
- Yan, C.Z.Y.; Austin, C.M.; Ayub, Q.; Rahman, S.; Gan, H.M. Genomic characterization of Vibrio parahaemolyticus from Pacific white shrimp and rearing water in Malaysia reveals novel sequence types and structural variation in genomic regions containing the Photorhabdus insect-related (Pir) toxin-like genes. FEMS Microbiol. Lett. 2019, 366, fnz211. [Google Scholar] [CrossRef]
- Waters, V.L. Conjugative transfer in the dissemination of beta-lactam and aminoglycoside resistance. Front. Biosci. 1999, 4, D433–D456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cabezón, E.; de la Cruz, F.; Arechaga, I. Conjugation inhibitors and their potential use to prevent dissemination of antibiotic resistance genes in bacteria. Front. Microbiol. 2017, 8, 2329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ripoll-Rozada, J.; Garcia-Cazorla, Y.; Getino, M.; Machon, C.; Sanabria-Rios, D.; de la Cruz, F.; Cabezón, E.; Arechaga, I. Type IV traffic ATPase TrwD as molecular target to inhibit bacterial conjugation. Mol. Microbiol. 2016, 100, 912–921. [Google Scholar] [CrossRef] [PubMed]
- Oyedemi, B.O.; Shinde, V.; Shinde, K.; Kakalou, D.; Stapleton, P.D.; Gibbons, S. Novel R-plasmid conjugal transfer inhibitory and antibacterial activities of phenolic compounds from Mallotus philippensis (Lam.) Mull. Arg. J. Glob. Antimicrob. Resist. 2016, 5, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Vasu, K.; Nagaraja, V. Diverse functions of restriction-modification systems in addition to cellular defense. Microbiol. Mol. Biol. Rev. 2013, 77, 53–72. [Google Scholar] [CrossRef] [Green Version]
- Wilkins, B.M. Plasmid promiscuity: Meeting the challenge of DNA immigration control. Environ. Microbiol. 2002, 4, 495–500. [Google Scholar] [CrossRef]
- Belogurov, A.A.; Delver, E.P. A motif conserved among the type I restriction-modification enzymes and antirestriction proteins: A possible basis for mechanism of action of plasmid-encoded antirestriction functions. Nucleic Acids Res. 1995, 23, 785–787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McMahon, S.A.; Roberts, G.A.; Johnson, K.A.; Cooper, L.P.; Liu, H.; White, J.H.; Carter, L.G.; Sanghvi, B.; Oke, M.; Walkinshaw, M.D.; et al. Extensive DNA mimicry by the ArdA anti-restriction protein and its role in the spread of antibiotic resistance. Nucleic Acids Res. 2009, 37, 4887–4897. [Google Scholar] [CrossRef]
- Serfiotis-Mitsa, D.; Herbert, A.P.; Roberts, G.A.; Soares, D.C.; White, J.H.; Blakely, G.W.; Uhrín, D.; Dryden, D.T.F. The structure of the KlcA and ArdB proteins reveals a novel fold and antirestriction activity against Type I DNA restriction systems in vivo but not in vitro. Nucleic Acids Res. 2010, 38, 1723–1737. [Google Scholar] [CrossRef] [Green Version]
- Balabanov, V.P.; Pustovoit, K.S.; Zavilgelsky, G.B. Comparative analysis of antirestriction activity of the ArdA and ArdB proteins encoded by genes of the R64 transmissible plasmid (IncI1). Mol. Biol. 2012, 46, 244–249. [Google Scholar] [CrossRef]
- Wang, H.C.; Ho, C.H.; Hsu, K.C.; Yang, J.M.; Wang, A.H.J. DNA mimic proteins: Functions, structures and bioinformatic analysis. Biochemistry 2014, 42, 1354–1364. [Google Scholar] [CrossRef] [Green Version]
- Henke, J.M.; Bassler, B.L. Quorum sensing regulates type III secretion in Vibrio harveyi and Vibrio parahaemolyticus. J. Bacteriol. 2004, 186, 3794–3805. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Zhou, D.; Mao, P.; Zhang, Y.; Hou, J.; Hu, Y.; Li, J.; Hou, S.; Yang, R.; Wang, R.; et al. Cell density- and quorum sensing-dependent expression of type VI secretion system 2 in Vibrio parahaemolyticus. PLoS ONE 2013, 8, e73363. [Google Scholar] [CrossRef] [Green Version]
- Park, K.S.; Ono, T.; Rokuda, M.; Jang, M.H.; Okada, K.; Iida, T.; Honda, T. Functional characterization of two type III secretion systems of Vibrio parahaemolyticus. Infect. Immun. 2004, 72, 6659–6665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, P.; Kinch, L.N.; Ray, A.; Dalia, A.B.; Cong, Q.; Nunan, L.M.; Camilli, A.; Grishin, N.V.; Salomon, D.; Orth, K. Acute hepatopancreatic necrosis disease-causing Vibrio parahaemolyticus strains maintain an antibacterial type VI secretion system with versatile effector repertoires. Appl. Environ. Microbiol. 2017, 83, e00737-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, Y.; Yang, H.; Li, J.; Zhang, P.; Wu, B.; Zhu, B.; Zhang, Y.; Fang, W. Putative type VI secretion systems of Vibrio parahaemolyticus contribute to adhesion to cultured cell monolayers. Arch. Microbiol. 2012, 194, 827–835. [Google Scholar] [CrossRef] [PubMed]
- Filloux, A. Protein secretion systems in Pseudomonas aeruginosa: An essay on diversity, evolution, and function. Front. Microbiol. 2011, 2, 155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gu, L.; Zhou, S.; Zhu, L.; Liang, C.; Chen, X. Small-molecule inhibitors of the type III secretion system. Molecules 2015, 20, 17659–17674. [Google Scholar] [CrossRef] [PubMed]
- Waack, U.; Johnson, T.L.; Chedid, K.; Xi, C.; Simmons, L.A.; Mobley, H.; Sandkvist, M. Targeting the type II secretion system: Development, optimization, and validation of a high-throughput screen for the identification of small molecule inhibitors. Front. Cell Infect. Microbiol. 2017, 7, 380. [Google Scholar] [CrossRef]
- Nielsen, A.K.; Thorsted, P.; Thisted, T.; Wagner, E.G.; Gerdes, K. The rifampicin-inducible genes srnB from F and pnd from R483 are regulated by antisense RNAs and mediate plasmid maintenance by killing of plasmid-free segregants. Mol. Microbiol. 1991, 5, 1961–1973. [Google Scholar] [CrossRef]
- Thisted, T.; Nielsen, A.K.; Gerdes, K. Mechanism of post-segregational killing: Translation of Hok, SrnB and Pnd mRNAs of plasmids R1, F and R483 is activated by 3′-end processing. EMBO J. 1994, 13, 1950–1959. [Google Scholar] [CrossRef]
- Palmer, B.R.; Marinus, M.G. The dam and dcm strains of Escherichia coli—A review. Gene 1994, 143, 1–12. [Google Scholar] [CrossRef]
- Kobayashi, I.; Nobusato, A.; Kobayashi-Takahashi, N.; Uchiyama, I. Shaping the genome--restriction-modification systems as mobile genetic elements. Curr. Opin. Genet. Dev. 1999, 9, 649–656. [Google Scholar] [CrossRef]
- Roberts, R.J.; Macelis, D. REBASE—restriction enzymes and methylases. Nucleic Acids Res. 2000, 28, 306–307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Low, D.A.; Weyand, N.J.; Mahan, M.J. Roles of DNA adenine methylation in regulating bacterial gene expression and virulence. Infect. Immun. 2001, 69, 7197–7204. [Google Scholar] [CrossRef] [Green Version]
- Torreblanca, J.; Marqués, S.; Casadesús, J. Synthesis of FinP RNA by plasmids F and pSLT is regulated by DNA adenine methylation. Genetics 1999, 152, 31–45. [Google Scholar]


| Pore Forming Toxins (2 †) | ||||
| ORF | Gene/Protein Name | Accession No. * | Location and Direction | Function Annotation # and Related References |
| ORF50 | PirBvp | AKC05670 | 33699←35015 | Photorhabdus insect-related (Pir) B; delta endotoxin, N-terminal domain [5,9] |
| ORF51 | PirAvp | AKC05671 | 35028←35363 | Photorhabdus insect-related (Pir) A [5,9] |
| Transposition (5 †) | ||||
| ORF | Gene/Protein Name | Accession No. * | Location and Direction | Function Annotation # |
| ORF15 | Transposase | AKC05635 | 9224→10144 | IS5 family transposase; DDE domain transposase [26] |
| ORF48 | Transposase | AKC05668 | 32035←32955 | IS5 family transposase; DDE domain transposase [26] |
| ORF55 | Transposase | AKC05675 | 36541→37461 | IS5 family transposase; DDE domain transposase [26] |
| ORF57 | Transposase | AKC05677 | 38476→38607 | Transposase zinc-binding domain; NCBI conserved domain: Zn_Tnp_IS91 (pfam14319) [27] |
| ORF68 | Transposase | AKC05688 | 48532→49476 | Rpn family recombination-promoting nuclease/putative transposase [28] |
| Secretion System (4 †) | ||||
| ORF | Gene/Protein Name | Accession No. * | Location and Direction | Function Annotation # |
| ORF3 | Type IV pilin protein | AKC05623 | 1052→2011 | Type II secretory pathway, pseudopilin PulG (Cell motility, Intracellular trafficking, secretion, and vesicular transport, Extracellular structures) [29] |
| ORF86 | Type II and III secretion system protein | AKC05706 | 64495→65997 | Pilus (MSHA, mannose-sensitive hemagglutinin type) biogenesis protein MshL; Bacterial type II and III secretion system protein [29] |
| ORF89 | Type II secretion protein | AKC05709 | 67759→69381 | Type II secretion protein; P-loop containing Nucleoside Triphosphate Hydrolases; Walker A motif; ATP binding site (chemical binding); Walker B motif [29] |
| ORF90 | Type II secretion protein F | AKC05710 | 69378→70382 | Type II secretion protein F [29] |
| Conjugation Factor (11 †) | ||||
| ORF | Gene/Protein Name | Accession No. * | Location and Direction | Function Annotation # |
| ORF10 | TraF | AKC05630 | 5999→6511 | Conjugative transfer signal peptidase TraF; Signal peptidase, peptidase S26 [30,31] |
| ORF11 | VirB1-like conjugal transfer protein | AKC05631 | 6525→7040 | Conjugal transfer protein; VirB1-like subfamily; one of twelve proteins making up type IV secretion systems (T4SS); VirB1 transglycosylase activity facilitate T4SS assembly [32] |
| ORF74 | TrbC | AKC05694 | 54020→54349 | Conjugal transfer protein TrbC; TrbC/VIRB2 family [30,31] |
| ORF75 | TrbD | AKC05695 | 54360→54674 | Mating pair formation protein TrbD; Type IV secretory pathway, VirB3-like protein [30,31] |
| ORF76 | TrbE | AKC05696 | 54715→57129 | Mating pair formation protein TrbE; conjugal transfer protein TrbE; Provisional; CagE, TrbE, VirB family, component of type IV transporter system; P-loop containing nucleoside triphosphate hydrolases [30,31] |
| ORF78 | TrbB | AKC05698 | 57895→58842 | Conjugal transfer protein TrbB; P-loop containing Nucleoside Triphosphate Hydrolases [30,31] |
| ORF79 | TrbL | AKC05699 | 58839→60149 | Conjugal transfer protein; TrbL/VirB6 plasmid conjugal transfer protein [30,31] |
| ORF81 | TrbF | AKC05701 | 60380→61054 | Conjugal transfer protein TrbF; VirB8 protein; putative dimerization motif (polypeptide binding) [30,31] |
| ORF82 | TrbG | AKC05702 | 61063→61935 | Conjugal transfer protein TrbG; VirB9/CagX/TrbG, a component of the type IV secretion system; VirB7 interaction site [30,31] |
| ORF83 | TrbH | AKC05703 | 61935→62357 | Conjugal transfer protein TrbH [30,31] |
| ORF84 | TrbI | AKC05704 | 62350→63606 | TrbI; conjugal transfer protein TrbI; Provisional; Bacterial conjugation TrbI-like protein [30,31] |
| Antirestriction Protein (2 †) | ||||
| ORF | Gene/Protein Name | Accession No. * | Location and Direction | Function Annotation # |
| ORF32 | Antirestriction protein (ArdB) | AKC05652 | 21587←22051 | Antirestriction protein; ArdB [33] |
| ORF35 | DNA primase; antirestriction protein (ArdC) | AKC05655 | 22595←23488 | DNA primase; antirestriction protein (ArdC) [34] |
| Post-Segregational Killing System(1 †) | ||||
| ORF | Gene/protein Name | Accession No. * | Location and Direction | Function Annotation # |
| ORF7 | Protein hokC /pndA | AKC05627 | 4292→4444 | Hok/gef family; Associated with a post-segregational killing (PSK) system [35] |
| DNA methyltransferase (2 †) | ||||
| ORF | Gene/Protein Name | Accession No. * | Location and Direction | Function Annotation # |
| ORF47 | N-6 DNA Methylase family protein | AKC05667 | 30063←32042 | N-6 DNA Methylase family protein [36] |
| ORF56 | Methylase family protein | AKC05676 | 37446←37976 | Methylase family protein [36] |
| Known Function (15 †) | ||||
| ORF | Gene/Protein Name | Accession No. * | Location and Direction | Function Annotation # |
| ORF5 | Trypsin protease | AKC05625 | 2553→3638 | Trypsin-like protease; NCBI conserved domain: Trypsin_2 (pfam13365) [27] |
| ORF21 | DNA-binding protein | AKC05641 | 12652→13059 | Domain in histone-like proteins of HNS family; NCBI conserved domain: Histone_HNS (pfam00816) [27] |
| ORF23 | MobB | AKC05643 | 14053→16020 | Molybdopterin-guanine dinucleotide biosynthesis protein MobB; P-loop containing nucleoside triphosphate hydrolases [37] |
| ORF26 | Mobilization protein | AKC05646 | 16788→17528 | Mobilization protein [37] |
| ORF27 | DNA topoisomerase III | AKC05647 | 17595→19805 | DNA topoisomerase III. DNA Topoisomerase, subtype IA; DNA-binding, ATP-binding and catalytic domain of bacterial DNA topoisomerases I and III, and eukaryotic DNA topoisomerase III and eubacterial and archael reverse gyrases [38] |
| ORF40 | DNA-binding protein | AKC05660 | 24974←25270 | Putative DNA-binding protein (no available reference) |
| ORF46 | SSU ribosomal protein S2p | AKC05666 | 28045←29535 | SSU (small subunit of ribosome) ribosomal protein S2p [39] |
| ORF53 | Exported protein | AKC05673 | 35657←36133 | Exported protein of unknown function [no available reference] |
| ORF61 | Recombinase RecF | AKC05681 | 40846→43179 | DNA repair exonuclease SbcCD ATPase subunit [Replication, recombination and repair] [40] |
| ORF62 | Phosphatidylinositol kinase | AKC05682 | 43568→44392 | Phosphatidylinositol kinase, CoiA-like protein [41] |
| ORF63 | Cold-shock protein | AKC05683 | 44809←45483 | Ribosomal protein S1-like RNA-binding domain. Found in a wide variety of RNA-associated proteins; NCBI conserved domain: CSP_CDS (cd04458) [27] |
| ORF64 | Alpha-helical coiled coil protein | AKC05684 | 45537←46523 | SMC_prok_B; chromosome segregation protein SMC, common bacterial type; NCBI conserved domains: KfrA_N (pfam11740) and CCDC158 (pfam15921) [27] |
| ORF66 | Tyrosine-tRNA ligase | AKC05686 | 47258←47578 | Putative tyrosine-tRNA ligase [no available reference] |
| ORF67 | ParA | AKC05687 | 47562←48239 | Chromosome partitioning protein ParA; plasmid-partitioning protein RepA [42] |
| ORF71 | Rep | AKC05691 | 51712←52551 | RNA polymerase Rpb2, domain 4; NCBI conserved domain: RNA_pol_Rpb2_4 (pfam04566) [27] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.-C.; Lin, S.-J.; Mohapatra, A.; Kumar, R.; Wang, H.-C. A Review of the Functional Annotations of Important Genes in the AHPND-Causing pVA1 Plasmid. Microorganisms 2020, 8, 996. https://doi.org/10.3390/microorganisms8070996
Wang H-C, Lin S-J, Mohapatra A, Kumar R, Wang H-C. A Review of the Functional Annotations of Important Genes in the AHPND-Causing pVA1 Plasmid. Microorganisms. 2020; 8(7):996. https://doi.org/10.3390/microorganisms8070996
Chicago/Turabian StyleWang, Hao-Ching, Shin-Jen Lin, Arpita Mohapatra, Ramya Kumar, and Han-Ching Wang. 2020. "A Review of the Functional Annotations of Important Genes in the AHPND-Causing pVA1 Plasmid" Microorganisms 8, no. 7: 996. https://doi.org/10.3390/microorganisms8070996