Shape Morphable Hydrogel/Elastomer Bilayer for Implanted Retinal Electronics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fabrication of PDMS Elastomer
2.3. Fabrication of PDMS/Hydrogel Bilayer
2.4. Mechanical Test
2.5. Image Analysis
3. Results
3.1. Fabrication of Hydrogel Bilayer
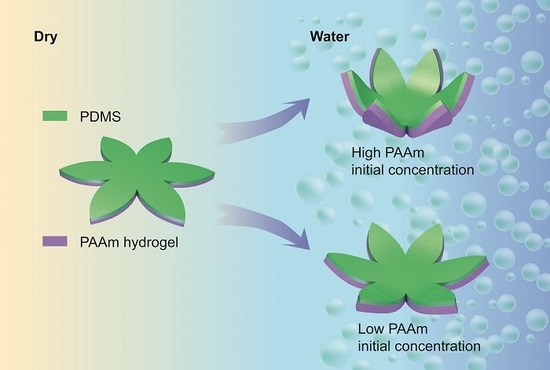
3.2. Initial Monomer Concentration Is Proportional to Final Bilayer Curvature
3.3. PAAm/PDMS Bilayer Can Be Retinal Array Substrate
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A

References
- Montero De Espinosa, L.; Meesorn, W.; Moatsou, D.; Weder, C. Bioinspired Polymer Systems with Stimuli-Responsive Mechanical Properties. Chem. Rev. 2017, 117, 12851–12892. [Google Scholar] [CrossRef]
- Ionov, L. Bioinspired microorigami by self-folding polymer films. Macromol. Chem. Phys. 2013, 214, 1178–1183. [Google Scholar] [CrossRef]
- Stuart, M.A.C.; Huck, W.T.S.; Genzer, J.; Müller, M.; Ober, C.; Stamm, M.; Sukhorukov, G.B.; Szleifer, I.; Tsukruk, V.V.; Urban, M.; et al. Emerging applications of stimuli-responsive polymer materials. Nat. Mater. 2010, 9, 101–113. [Google Scholar] [CrossRef]
- Ionov, L. Actuating Hydrogel Thin Film. In Responsive Polymer Surfaces: Dynamics in Surface Topography; Liu, D., Broer, D.J., Eds.; Wiley: Hoboken, NJ, USA, 2017; pp. 137–157. [Google Scholar] [CrossRef]
- Gracias, D.H. Stimuli responsive self-folding using thin polymer films. Curr. Opin. Chem. Eng. 2013, 2, 112–119. [Google Scholar] [CrossRef]
- Xu, W.; Qin, Z.; Chen, C.T.; Kwag, H.R.; Ma, Q.; Sarkar, A.; Buehler, M.J.; Gracias, D.H. Ultrathin thermoresponsive self-folding 3D graphene. Sci. Adv. 2017, 3, e1701084. [Google Scholar] [CrossRef] [Green Version]
- Stoychev, G.; Puretskiy, N.; Ionov, L. Self-folding all-polymer thermoresponsive microcapsules. Soft Matter 2011, 7, 3277–3279. [Google Scholar] [CrossRef]
- Vannozzi, L.; Yasa, I.C.; Ceylan, H.; Menciassi, A.; Ricotti, L.; Sitti, M. Self-Folded Hydrogel Tubes for Implantable Muscular Tissue Scaffolds. Macromol. Biosci. 2018, 18, 1–9. [Google Scholar] [CrossRef]
- Li, H.; Go, G.; Ko, S.Y.; Park, J.O.; Park, S. Magnetic actuated pH-responsive hydrogel-based soft micro-robot for targeted drug delivery. Smart Mater. Struct. 2016, 25, 027001. [Google Scholar] [CrossRef]
- Li, X.; Cai, X.; Gao, Y.; Serpe, M.J. Reversible bidirectional bending of hydrogel-based bilayer actuators. J. Mater. Chem. B 2017, 5, 2804–2812. [Google Scholar] [CrossRef]
- Magdanz, V.; Stoychev, G.; Ionov, L.; Sanchez, S.; Schmidt, O.G. Stimuli-responsive microjets with reconfigurable shape. Angew. Chem. Int. Ed. 2014, 53, 2673–2677. [Google Scholar] [CrossRef]
- Ma, C.; Lu, W.; Yang, X.; He, J.; Le, X.; Wang, L.; Zhang, J.; Serpe, M.J.; Huang, Y.; Chen, T. Bioinspired Anisotropic Hydrogel Actuators with On–Off Switchable and Color-Tunable Fluorescence Behaviors. Adv. Funct. Mater. 2018, 28, 1704568. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, Y.; Wu, B.; Sun, W.; Li, Z.; Sun, J. Polyelectrolyte multilayer films for building energetic walking devices. Angew. Chem. Int. Ed. 2011, 50, 6254–6257. [Google Scholar] [CrossRef] [PubMed]
- Macron, J.; Gerratt, A.P.; Lacour, S.P. Thin Hydrogel–Elastomer Multilayer Encapsulation for Soft Electronics. Adv. Mater. Technol. 2019, 4, 1900331. [Google Scholar] [CrossRef] [Green Version]
- Ware, T.; Simon, D.; Hearon, K.; Liu, C.; Shah, S.; Reeder, J.; Khodaparast, N.; Kilgard, M.P.; Maitland, D.J.; Rennaker, R.L.; et al. Three-dimensional flexible electronics enabled by shape memory polymer substrates for responsive neural interfaces. Macromol. Mater. Eng. 2012, 297, 1193–1202. [Google Scholar] [CrossRef]
- Zhang, Y.; Zheng, N.; Cao, Y.; Wang, F.; Wang, P.; Ma, Y.; Lu, B.; Hou, G.; Fang, Z.; Liang, Z.; et al. Climbing-inspired twining electrodes using shape memory for peripheral nerve stimulation and recording. Sci. Adv. 2019, 5, eaaw1066. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Zhao, Q.; Wang, Y.; Zeng, Q.; Wu, T.; Du, X. Shape-Programmable Electronics: Self-Unfolding Flexible Microelectrode Arrays Based on Shape Memory Polymers (Adv. Mater. Technol. 11/2019). Adv. Mater. Technol. 2019, 4, 1970063. [Google Scholar] [CrossRef]
- Yu, C.; Duan, Z.; Yuan, P.; Li, Y.; Su, Y.; Zhang, X.; Pan, Y.; Dai, L.L.; Nuzzo, R.G.; Huang, Y.; et al. Electronically programmable, reversible shape change in two- and three-dimensional hydrogel structures. Adv. Mater. 2013, 25, 1541–1546. [Google Scholar] [CrossRef]
- Li, X.; Lin, S.; Liang, J.; Zhang, Y.; Oigawa, H.; Ueda, T. Fiber-optic temperature sensor based on difference of thermal expansion coefficient between fused silica and metallic materials. IEEE Photonics J. 2012, 4, 155–162. [Google Scholar]
- Ravindran, S.K.T.; Kroener, M.; Woias, P. A bimetallic micro heat engine for pyroelectric energy conversion. Procedia Eng. 2012, 47, 33–36. [Google Scholar] [CrossRef] [Green Version]
- White, E.M.; Yatvin, J.; Grubbs, J.B.; Bilbrey, J.A.; Locklin, J. Advances in smart materials: Stimuli-responsive hydrogel thin films. J. Polym. Sci. Part B Polym. Phys. 2013, 51, 1084–1099. [Google Scholar] [CrossRef]
- Kim, J.; Kim, C.; Song, Y.S.; Jeong, S.G.; Kim, T.S.; Lee, C.S. Reversible self-bending soft hydrogel microstructures with mechanically optimized designs. Chem. Eng. J. 2017, 321, 384–393. [Google Scholar] [CrossRef]
- Stoychev, G.; Zakharchenko, S.; Turcaud, S.; Dunlop, J.W.C.; Ionov, L. Shape-programmed folding of stimuli-responsive polymer bilayers. ACS Nano 2012, 6, 3925–3934. [Google Scholar] [CrossRef] [Green Version]
- Baker, J.P.; Hong, L.H.; Blanch, H.W.; Prausnitz, J.M. Effect of Initial Total Monomer Concentration on the Swelling Behavior of Cationic Acrylamide-Based Hydrogels. Macromolecules 1994, 27, 1446–1454. [Google Scholar] [CrossRef] [Green Version]
- Baselga, J.; Hernández-Fuentes, I.; Piérola, I.F.; Llorente, M.A. Elastic Properties of Highly Cross-Linked Polyacrylarnide Gels. Macromolecules 1987, 20, 3060–3065. [Google Scholar] [CrossRef] [Green Version]
- Weiss, N.; Van Vliet, T.; Silberberg, A. Influence of Polymerization Initiation Rate on Permeability of Aqueous Polyacrylamide Gels. J. Polym. Sci. Part A2 Polym. Phys. 1981, 19, 1505–1512. [Google Scholar] [CrossRef]
- Sheth, S.; Jain, E.; Karadaghy, A.; Syed, S.; Stevenson, H.; Zustiak, S.P. UV Dose Governs UV-Polymerized Polyacrylamide Hydrogel Modulus. Int. J. Polym. Sci. 2017, 6, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Second Sight Medical Products Inc. Argus II Retinal Prosthesis System Surgeon Manual; Second Sight Medical Products Inc.: Sylmar, CA, USA, 2013. [Google Scholar]
- Waschkowski, F.; Hesse, S.; Rieck, A.C.; Lohmann, T.; Brockmann, C.; Laube, T.; Bornfeld, N.; Thumann, G.; Walter, P.; Mokwa, W.; et al. Development of very large electrode arrays for epiretinal stimulation (VLARS). Biomed. Eng. Online 2014, 13, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Ameri, H.; Ratanapakorn, T.; Ufer, S.; Eckhardt, H.; Humayun, M.S.; Weiland, J.D. Toward a wide-field retinal prosthesis. J. Neural Eng. 2009, 6, 035002. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Zhao, Q.; Wang, Y.; Zeng, Q.; Wu, T.; Du, X. Self-Unfolding Flexible Microelectrode Arrays Based on Shape Memory Polymers. Adv. Mater. Technol. 2019, 4, 1900566. [Google Scholar] [CrossRef]
- Ferlauto, L.; Airaghi Leccardi, M.J.I.; Chenais, N.A.L.; Gilliéron, S.C.A.; Vagni, P.; Bevilacqua, M.; Wolfensberger, T.J.; Sivula, K.; Ghezzi, D. Design and validation of a foldable and photovoltaic wide-field epiretinal prosthesis. Nat. Commun. 2018, 9, 992. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Park, J.; Lang, R.J.; Emami-Neyestanak, A.; Pellegrino, S.; Humayun, M.S.; Tai, Y.C. Parylene origami structure for introcular implantation. In Proceedings of the 2013 Transducers Eurosensors XXVII 17th International Conference Solid-State Sensors, Actuators Microsystems, Transducers Eurosensors, Barcelona, Spain, 16–20 June 2013; pp. 1549–1552. [Google Scholar]
- Yuk, H.; Zhang, T.; Parada, G.A.; Liu, X.; Zhao, X. Skin-inspired hydrogel-elastomer hybrids with robust interfaces and functional microstructures. Nat. Commun. 2016, 7, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Fujie, T.; Desii, A.; Ventrelli, L.; Mazzolai, B.; Mattoli, V. Inkjet printing of protein microarrays on freestanding polymeric nanofilms for spatio-selective cell culture environment. Biomed. Microdevices 2012, 14, 1069–1076. [Google Scholar] [CrossRef] [PubMed]
- Minev, I.R.; Musienko, P.; Hirsch, A.; Barraud, Q.; Wenger, N.; Moraud, E.M.; Gandar, J.; Capogrosso, M.; Milekovic, T.; Asboth, L.; et al. Electronic dura mater for long-term multimodal neural interfaces. Science (80-) 2015, 347, 159–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alam, S.; Khan, G.M.A.; Razzaque, S.M.A.; Alam, M.N.; Mondal, M.I.H. Swelling property of the polyacrylamide hydrogel prepared by γ-ray irradiation. J. Polym. Mater. 2008, 25, 645–651. [Google Scholar]
- Orakdogen, N.; Okay, O. Effect of initial monomer concentration on the equilibrium swelling and elasticity of hydrogels. Eur. Polym. J. 2006, 42, 955–960. [Google Scholar] [CrossRef]
- Kizilay, M.Y.; Okay, O. Effect of initial monomer concentration on spatial inhomogeneity in poly(acrylamide) gels. Macromolecules 2003, 36, 6856–6862. [Google Scholar] [CrossRef]
- Timoshenko, B.Y.S. Analysis of bi-metal thermostats. JOSA 1925, 11, 233–255. [Google Scholar] [CrossRef]
- Liu, S.; Boatti, E.; Bertoldi, K.; Kramer-Bottiglio, R. Stimuli-induced bi-directional hydrogel unimorph actuators. Extrem. Mech. Lett. 2018, 21, 35–43. [Google Scholar] [CrossRef]
- Guo, W.; Li, M.; Zhou, J. Modeling programmable deformation of self-folding all-polymer structures with temperature-sensitive hydrogels. Smart Mater. Struct. 2013, 22, 115028. [Google Scholar] [CrossRef]
- Reinhard Rüchel, M.D.B. Scanning electron microscopic observations of polyacrylamide gels. Anal. Biochem. 1975, 68, 415–428. [Google Scholar] [CrossRef]
- Lovie-Kitchin, J.E.; Soonng, G.P.; Hassan, S.E.; Woods, R.L. Visual Field Size Criteria for Mobility. Optom. Vis. Sci. 2010, 87, 948–957. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhattacharya, S.S.; Chakarova, C.F. Retinitis Pigmentosa. In Brenner’s Encyclopedia Genetic, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2013; Volume 12, pp. 199–203. [Google Scholar]
- Humayun, M.S.; Dorn, J.D.; Da Cruz, L.; Dagnelie, G.; Sahel, J.A.; Stanga, P.E.; Cideciyan, A.V.; Duncan, J.L.; Eliott, D.; Filley, E.; et al. Interim results from the international trial of second sight’s visual prosthesis. Ophthalmology 2012, 119, 779–788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, G.; Wang, Y.X.; Zheng, Z.Y.; Yang, H.; Xu, L.; Jonas, J.B. Ocular axial length and its associations in Chinese: The Beijing eye study. PLoS One 2012, 7, e43172. [Google Scholar] [CrossRef] [Green Version]
- Thiel, J.; Maurer, G. Swelling equilibrium of poly(acrylamide) gels in aqueous salt and polymer solutions. Fluid Phase Equilibria 1999, 165, 225–260. [Google Scholar] [CrossRef]
- Ahuja, A.K.; Yeoh, J.; Dorn, J.D.; Caspi, A.; Wuyyuru, V.; McMahon, M.J.; Humayun, M.S.; Greenberg, R.J.; daCruz, L. Argus II Study Group Factors Affecting Perceptual Threshold in Argus II Retinal Prosthesis Subjects. Transl. Vis. Sci. Technol. 2013, 2, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greenbaum, E.; Zhou, D. Implantable Neural Prostheses 1; Springer: New York, NY, USA, 2009. [Google Scholar]
- Petrossians, A.; Whalen III, J.J.; Weiland, J.D.; Mansfeld, F. Electrodeposition and Characterization of Thin-Film Platinum-Iridium Alloys for Biological Interfaces. J. Electrochem. Soc. 2011, 158, D269. [Google Scholar] [CrossRef]
- Cogan, S.F. Neural Stimulation and Recording Electrodes. Annu. Rev. Biomed. Eng. 2008, 10, 275–309. [Google Scholar] [CrossRef] [Green Version]
- De Balthasar, C.; Patel, S.; Roy, A.; Freda, R.; Greenwald, S.; Horsager, A.; Mahadevappa, M.; Yanai, D.; McMahon, M.J.; Humayun, M.S.; et al. Factors affecting perceptual thresholds in epiretinal prostheses. Investig. Ophthalmol. Vis. Sci. 2008, 49, 2303–2314. [Google Scholar] [CrossRef] [Green Version]
- Shamsudeen, R.K.; Nair, S.; Jayakumari, V.G. Equilibrium swelling, conductivity and electroactive characteristics of polyacrylamide hydrogels. Indian J. Eng. Mater. Sci. 2006, 13, 62–68. [Google Scholar]
- Chou, N.; Jeong, J.; Kim, S. Crack-free and reliable lithographical patterning methods on PDMS substrate. J. Micromech. Microeng. 2013, 23, 125035. [Google Scholar] [CrossRef]
- Bowden, N.; Brittain, S.; Evans, A.G.; Hutchinson, J.W.; Whitesides, G.M. Spontaneous formation of ordered structures in thin films of metals supported on an elastomeric polymer. Nature 1998, 393, 146–149. [Google Scholar] [CrossRef]
- Adrega, T.; Lacour, S.P. Stretchable gold conductors embedded in PDMS and patterned by photolithography: Fabrication and electromechanical characterization. J. Micromech. Microeng. 2010, 20, 055025. [Google Scholar] [CrossRef]




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, M.; Kang, D.H.; Kim, J.; Weiland, J.D. Shape Morphable Hydrogel/Elastomer Bilayer for Implanted Retinal Electronics. Micromachines 2020, 11, 392. https://doi.org/10.3390/mi11040392
Zhou M, Kang DH, Kim J, Weiland JD. Shape Morphable Hydrogel/Elastomer Bilayer for Implanted Retinal Electronics. Micromachines. 2020; 11(4):392. https://doi.org/10.3390/mi11040392
Chicago/Turabian StyleZhou, Muru, Do Hyun Kang, Jinsang Kim, and James D. Weiland. 2020. "Shape Morphable Hydrogel/Elastomer Bilayer for Implanted Retinal Electronics" Micromachines 11, no. 4: 392. https://doi.org/10.3390/mi11040392