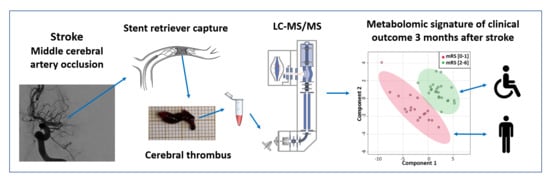
Metabolome of Cerebral Thrombi Reveals an Association between High Glycemia at Stroke Onset and Good Clinical Outcome
Abstract
:1. Introduction
2. Results
2.1. Patient Characteristics
2.2. Composition of Cerebral Thrombi Assessed by Proteomic Analyses
2.3. Untargeted Metabolomic Analyses of Cerebral Thrombi
2.4. Analyses of the Polyol (Sorbitol) Pathway in Cerebral Thrombi Cells Assessed by Metabolomic and Proteomic Analyses
2.5. Predictive Value Comparison between Sorbitol in Cerebral Thrombi Reflecting Glycemia at Stroke Onset and Admission Glycemia
3. Discussion
4. Materials and Methods
4.1. Patient Selection and Procedure
4.2. Collection and Processing of Cerebral Thrombi for Omic Analyses
4.3. Metabolomic Analyses
4.4. Proteomic Analysis
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Goyal, M.; Menon, B.K.; Van Zwam, W.H.; Dippel, D.W.J.; Mitchell, P.J.; Demchuk, A.M.; Dávalos, A.; Majoie, C.B.L.M.; Van Der Lugt, A.; De Miquel, M.A.; et al. Endovascular thrombectomy after large-vessel ischaemic stroke: A meta-analysis of individual patient data from five randomised trials. Lancet 2016, 387, 1723–1731. [Google Scholar] [CrossRef]
- Powers, W.J.; Rabinstein, A.A.; Ackerson, T.; Adeoye, O.M.; Bambakidis, N.C.; Becker, K.; Biller, J.; Brown, M.; Demaerschalk, B.M.; Hoh, B.; et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: A guideline for healthcare professionals from the american heart association/american stroke association. Stroke 2019, 50, e344–e418. [Google Scholar]
- Maas, M.B.; Kapinos, G. Recanalization for large vessel stroke: Necessary but not sufficient for reperfusion. Neurology 2019, 92, 1127–1128. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, M.; Hao, Y.; Zi, W.; Yang, N.; Zhou, Z.; Geng, Y.; Wang, Z.; Li, H.; Xu, G.; et al. Early prediction of poor outcome despite successful recanalization after endovascular treatment for anterior large vessel occlusion stroke. World Neurosurg. 2018, 115, e312–e321. [Google Scholar] [CrossRef]
- Chamorro, Á.; Brown, S.; Amaro, S.; Hill, M.D.; Muir, K.W.; Dippel, D.W.; Van Zwam, W.; Butcher, K.; Ford, G.A.; Hertog, H.M.D.; et al. Glucose modifies the effect of endovascular thrombectomy in patients with acute stroke. Stroke 2019, 50, 690–696. [Google Scholar] [CrossRef] [Green Version]
- Robbins, N.M.; Swanson, R.A. Opposing effects of glucose on stroke and reperfusion injury: Acidosis, oxidative stress, and energy metabolism. Stroke 2014, 45, 1881–1886. [Google Scholar] [CrossRef] [Green Version]
- Luitse, A.M.J.; Biessels, G.J.; Rutten, G.E.H.M.; Kappelle, L.J. Diabetes, hyperglycaemia, and acute ischaemic stroke. Lancet Neurol. 2012, 11, 261–271. [Google Scholar] [CrossRef]
- Bacigaluppi, M.; Semerano, A.; Gullotta, G.S.; Strambo, D. Insights from thrombi retrieved in stroke due to large vessel occlusion. Br. J. Pharmacol. 2019, 39, 1433–1451. [Google Scholar] [CrossRef]
- Malone, J.I.; Leavengood, H.; Peterson, M.J.; O’brien, M.M.; Page, M.G. Red blood cell sorbitol as an indicator of polyol pathway activity. Diabetes 1984, 33, 45–49. [Google Scholar] [CrossRef]
- Malone, J.I.; Knox, G.; Benford, S.; Tedesco, T.A. Red cell sorbitol: An indicator of diabetic control. Diabetes 1980, 29, 861–864. [Google Scholar] [CrossRef]
- Steinberg, M.P. Red cell sorbitol an indicator of glycemic control. Pediatr. Res. 1977, 11, 519. [Google Scholar] [CrossRef] [Green Version]
- Nagasaka, Y.; Fujii, S.; Kaneko, T. Human erythrocyte sorbitol metabolism and the role of sorbitol dehydrogenase. Diabetologia 1988, 31, 766–770. [Google Scholar] [CrossRef] [Green Version]
- Wagner, K.R.; Kleinholz, M.; De Courten-Myers, G.M.; Myers, R.E. Hyperglycemic versus normoglycemic stroke: Topography of brain metabolites, intracellular pH, and infarct size. Br. J. Pharmacol. 1992, 12, 213–222. [Google Scholar] [CrossRef] [Green Version]
- Siesjö, B.K. Acidosis and ischemic brain damage. Neurochem. Pathol. 1988, 9, 58. [Google Scholar]
- Piironen, K.; Putaala, J.; Rosso, C.; Samson, Y. Glucose and acute stroke: Evidence for an interlude. Stroke 2012, 43, 898–902. [Google Scholar] [CrossRef] [Green Version]
- Schurr, A. Bench-to-bedside review: A possible resolution of the glucose paradox of cerebral ischemia. Crit. Care 2002, 6, 330–334. [Google Scholar] [CrossRef]
- Montaner, J.; Molina, C.A.; Montaner, J.; Arenillas, J.F.; Huertas, R.; Ribo, M.; Codina, A.; Quintana, M. Effects of admission hyperglycemia on stroke outcome in reperfused tissue plasminogen activator–treated patients. Stroke 2003, 34, 1235–1240. [Google Scholar]
- Rosso, C.; Baronnet, F.; Diaz, B.; Le Bouc, R.; Polara, G.F.; Moulton, E.J.; Deltour, S.; Léger, A.; Crozier, S.; Samson, Y. The silver effect of admission glucose level on excellent outcome in thrombolysed stroke patients. J. Neurol. 2018, 265, 1684–1689. [Google Scholar] [CrossRef] [Green Version]
- Prado, R.; Ginsberg, M.D.; Dietrich, W.D.; Watson, B.D.; Busto, R. Hyperglycemia increases infarct size in collaterally perfused but not end-arterial vascular territories. J. Cereb. Blood Flow Metab. 1988, 8, 186–192. [Google Scholar] [CrossRef] [Green Version]
- Ntaios, G.; Papavasileiou, V.; Bargiota, A.; Makaritsis, K.; Michel, P. Intravenous insulin treatment in acute stroke: A systematic review and meta-analysis of randomized controlled trials. Int. J. Stroke 2014, 9, 489–493. [Google Scholar] [CrossRef]
- Rosso, C.; Colliot, O.; Valabrègue, R.; Crozier, S.; Dormont, D.; Lehéricy, S.; Samson, Y. Tissue at risk in the deep middle cerebral artery territory is critical to stroke outcome. Neuroradiology 2011, 53, 763–771. [Google Scholar] [CrossRef]
- Pluskal, T.; Castillo, S.; Villar-Briones, A.; Orešič, M. MZmine 2: Modular framework for processing, visualizing, and analyzing mass spectrometry-based molecular profile data. BMC Bioinform. 2010, 11, 395. [Google Scholar] [CrossRef] [Green Version]
- Chong, J.; Wishart, D.S.; Xia, J. Using MetaboAnalyst 4.0 for comprehensive and integrative metabolomics data analysis. Curr. Protoc. Bioinform. 2019, 68, e86. [Google Scholar] [CrossRef]





| Patient Characteristics | All Patients (n = 41) | mRS < 2 (n = 18) | mRS ≥ 2 (n = 23) | p | |
|---|---|---|---|---|---|
| Demographic characteristics | |||||
| Age, years | * | 74.8 ± 13.2 | 70.2 ± 16.7 | 78.3 ± 12.7 | 0.0247 |
| Gender, male | 17 (41.5%) | 5 (27.8%) | 12 (52.2%) | 0.2010 | |
| Medical history | |||||
| Hypertension | 17 (41.5%) | 6 (33.3%) | 11 (47.8%) | 0.5240 | |
| Diabetes mellitus | 4 (9.8%) | 1 (5.6%) | 3 (13.0%) | 0.6180 | |
| Coronary artery disease | 4 (9.8%) | 3 (16.7%) | 1 (4.4%) | 0.3030 | |
| Smoking | 6 (14.6%) | 4 (22.2%) | 2 (8.7%) | 0.3770 | |
| Atrial fibrillation | 29 (70.7%) | 12 (66.7%) | 17 (73.9%) | 0.7340 | |
| Clinical characteristics | |||||
| Baseline NIHSS score | * | 17 (15–22) | 15 (13–21) | 20 (16–22) | 0.0480 |
| Baseline blood glucose, mg/dL | * | 134 ± 32 | 125 ± 21 | 142 ± 38 | 0.0378 |
| Imaging characteristics | |||||
| Tandem ICA/MCA occlusion | 5 (12.2%) | 1 (5.6%) | 4 (17.4%) | 0.3630 | |
| MRI brain imaging | 34 (82.9%) | 15 (83.3%) | 19 (82.6%) | 1.0000 | |
| DWI-ASPECTS | 7 (7–8) | 8 (7–8) | 7 (6–7) | 0.1180 | |
| DWI-Volume, cm3 | 9.8 (5.2–20.6) | 8.8 (4.9–16.6) | 12.6 (5.8–27.5) | 0.2746 | |
| Treatment characteristics | |||||
| Intravenous thrombolysis | 21 (51.2%) | 10 (55.6%) | 11 (47.8%) | 0.7560 | |
| Time to recanalization, min | 252.4 ± 62.3 | 245.8 ± 49.4 | 257.5 ± 71.4 | 0.2689 |
| Components | Description | mRS (0–1) | mRS (2–6) | p Value |
|---|---|---|---|---|
| RBC | Glycophorin-A | 1.67 × 108 ± 2.70 × 107 | 1.35 × 108 ± 1.69 × 107 | 0.3345 |
| Platelets | Platelet glycoprotein Ib alpha-chain | 4.63 × 107 ± 1.12 × 107 | 4.66 × 107 ± 1.35 × 107 | 0.9884 |
| Integrin beta-3 | 9.81 × 108 ± 1.48 × 108 | 9.46 × 108 ± 1.85 × 108 | 0.8881 | |
| Platelet endothelial cell adhesion molecule | 1.70 × 108 ± 2.76 × 107 | 1.73 × 108 ± 3.90 × 107 | 0.9510 | |
| Leukocytes | Receptor-type tyrosine-protein phosphatase C | 2.00 × 107 ± 3.66 × 106 | 2.31 × 107 ± 4.74 × 106 | 0.6281 |
| Fibrin | Fibrinogen alpha chain | 5.49 × 108 ± 1.78 × 108 | 1.38 × 109 ± 4.32 × 108 | 0.1125 |
| Fibrinogen beta chain | 5.01 × 109 ± 1.18 × 109 | 5.41 × 109 ± 9.19 × 108 | 0.7889 | |
| Fibrinogen gamma chain | 6.43 × 109 ± 1.66 × 109 | 8.38 × 109 ± 1.49 × 109 | 0.3880 | |
| von Willebrand factor | von Willebrand factor | 5.94 × 107 ± 1.96 × 107 | 6.11 × 107 ± 1.48 × 107 | 0.9444 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suissa, L.; Guigonis, J.-M.; Graslin, F.; Doche, E.; Osman, O.; Chau, Y.; Sedat, J.; Lindenthal, S.; Pourcher, T. Metabolome of Cerebral Thrombi Reveals an Association between High Glycemia at Stroke Onset and Good Clinical Outcome. Metabolites 2020, 10, 483. https://doi.org/10.3390/metabo10120483
Suissa L, Guigonis J-M, Graslin F, Doche E, Osman O, Chau Y, Sedat J, Lindenthal S, Pourcher T. Metabolome of Cerebral Thrombi Reveals an Association between High Glycemia at Stroke Onset and Good Clinical Outcome. Metabolites. 2020; 10(12):483. https://doi.org/10.3390/metabo10120483
Chicago/Turabian StyleSuissa, Laurent, Jean-Marie Guigonis, Fanny Graslin, Emilie Doche, Ophélie Osman, Yves Chau, Jacques Sedat, Sabine Lindenthal, and Thierry Pourcher. 2020. "Metabolome of Cerebral Thrombi Reveals an Association between High Glycemia at Stroke Onset and Good Clinical Outcome" Metabolites 10, no. 12: 483. https://doi.org/10.3390/metabo10120483