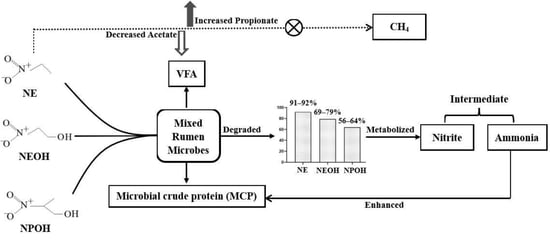
The Antimethanogenic Nitrocompounds Can be Cleaved into Nitrite by Rumen Microorganisms: A Comparison of Nitroethane, 2-Nitroethanol, and 2-Nitro-1-propanol
Abstract
:1. Introduction
2. Results
2.1. Effect of Nitrocompounds on VFA Production and Fermentation Gas Pattern
2.2. Disappearance Kinetics of NE, NEOH, and NPOH
2.3. Effect of Nitrocompounds on Nitrite Accumulation
2.4. Effect of Nitrocompounds on Ammonia N Production
2.5. Effect of Nitrocompounds on BCP Production
3. Discussion
3.1. Fermentation Characteristics and Antimethanogenic Activity of Nitrocompounds
3.2. Nitrocompounds’ Degradation and Metabolic Fate
3.3. Metabolic Fate of Nitrocompounds
4. Conclusions
5. Materials and Methods
5.1. Nitrocompounds
5.2. Animals and Inoculant
5.3. Substrate Preparation
5.4. Experimental Design
5.5. In Vitro Batch Culture and Sampling Procedure
5.6. Laboratory Analysis
5.7. Curve Fitting and Calculation
5.8. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
References
- Anderson, R.C.; Majak, W.; Rassmussen, M.A.; Callaway, T.R.; Beier, R.C.; Nisbet, D.J.; Allison, M.J. Toxicity and metabolism of the conjugates of 3-nitropropanol and 3-nitropropionic acid in forages poisonous to livestock. J. Agric. Food Chem. 2005, 53, 2344–2350. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.J.; Anderson, R.C. Toxicity and metabolism of nitroalkanes and substituted nitroalkanes. J. Agric. Food Chem. 2013, 61, 763–779. [Google Scholar] [CrossRef] [PubMed]
- Horrocks, S.M.; Jung, Y.S.; Huwe, J.K.; Harvey, R.B.; Ricke, S.C.; Carstens, G.E.; Callaway, T.R.; Anderson, R.C.; Ramlachan, N.; Nisbet, D.J. Inhibitory effects of short-chain nitrocompounds against Campylobacter jejuni and Campylobacter coli in vitro. J. Food Sci. 2007, 72, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.S.; Anderson, R.C.; Callaway, T.R.; Edrington, T.S.; Genovese, K.J.; Harvey, R.B.; Poole, T.L.; Nisbet, D.J. Inhibitory activity of 2-nitropropanol against select food-borne pathogens in vitro. Lett. Appl. Microbiol. 2004, 39, 471–476. [Google Scholar] [CrossRef]
- Anderson, R.C.; Huwe, J.K.; Smith, D.J.; Stanton, T.B.; Krueger, N.A.; Callaway, T.R.; Edrington, T.S.; Harvey, R.B.; Nisbet, D.J. Effect of nitroethane, dimethyl-2-nitroglutarate and 2-nitro-methyl-propionate on ruminal methane production and hydrogen balance in vitro. Bioresour. Technol. 2010, 101, 5345–5349. [Google Scholar] [CrossRef]
- Božic, A.K.; Anderson, R.C.; Carstens, G.E.; Ricke, S.C.; Callaway, T.R.; Yokoyama, M.T.; Wang, J.K.; Nisbet, D.J. Effects of the methane-inhibitors nitrate, nitroethane, lauric acid, Lauricidin and the Hawaiian marine algae. Bioresour. Technol. 2009, 99, 4017–4025. [Google Scholar] [CrossRef]
- Ochoa-Garcia, P.A.; Arevalos-Sanchez, M.M.; Ruiz-Barrera, O.; Anderson, R.C.; Maynez-Perez, A.O.; Rodriguez-Almeida, F.A.; Chavez-Martinez, A.; Gutierrez-Banuelos, H.; Corral-Luna, A. In vitro reduction of methane production by 3-nitro-1-propionic acid is dose-dependent1. J. Anim. Sci. 2019, 97, 1317–1324. [Google Scholar] [CrossRef]
- Zhang, D.F.; Yang, H.J. Combination effects of nitrocompounds, pyromellitic diimide, and 2-bromoethanesulfonate on in vitro ruminal methane production and fermentation of a grain-rich feed. J. Agric. Food Chem. 2012, 60, 364–371. [Google Scholar] [CrossRef]
- Gregory, N.G.; Orbell, G.M.; Harding, D.R. Poisoning with 3-nitropropionic acid in possums (Trichosurus vulpecula). N. Z. Vet. J. 2000, 48, 85–87. [Google Scholar] [CrossRef]
- Majak, W.; Pass, M.A. Aliphatic nitrocompounds. In Toxicants of Plant Origin, Vol. II, Glycosides; Cheeke, P.R., Ed.; CRC Press: Boca Raton, FL, USA, 1989; pp. 143–159. [Google Scholar]
- Anderson, R.C.; Rasmussen, M.A.; Allison, M.J. Metabolism of the plant toxins nitropropionic acid and nitropropanol by ruminal microorganisms. Appl. Environ. Microbiol. 1993, 59, 3056–3061. [Google Scholar]
- Nevel, C.J.V.; Demeyer, D.I. Control of rumen methanogenesis. Environ. Monit. Assess. 1996, 42, 73–97. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.C.; Rasmussen, M.A. Use of a novel nitrotoxin-metabolizing bacterium to reduce ruminal methane production. Bioresour. Technol. 1998, 64, 89–95. [Google Scholar] [CrossRef]
- Brown, E.G.; Anderson, R.C.; Carstens, G.E.; Gutierrez-Ba, U.H.; Mcreynolds, J.L.; Slay, L.J.; Callaway, T.R.; Nisbet, D.J. Effects of oral nitroethane administration on enteric methane emissions and ruminal fermentation in cattle. Anim. Feed Sci. Technol. 2011, 166–167, 275–281. [Google Scholar] [CrossRef]
- Morgavi, D.P.; Forano, E.; Martin, C.; Newbold, C.J. Microbial ecosystem and methanogenesis in ruminants. Animal 2010, 4, 1024–1036. [Google Scholar] [CrossRef] [Green Version]
- Niknam, V.; Ebrahimzadeh, H.; Maassoumi, A.A. Toxic nitro compounds in Astragalus species. Biochem. Syst. Ecol. 2003, 31, 557–562. [Google Scholar] [CrossRef]
- Gold, K.; Brodman, B.W. Studies on the distribution of a naturally occurring nitroaliphatic acid in crownvetch (Coronilla Varia, Fabaceae). Econ. Bot. 1991, 45, 334–338. [Google Scholar] [CrossRef]
- Majak, W.; Cheng, K.J. Identification of rumen bacteria that anaerobically degrade aliphatic nitrotoxins. Can. J. Microbiol. 1981, 27, 646–650. [Google Scholar] [CrossRef]
- Anderson, R.C.; Rasmussen, M.A.; Allison, M.J. Enrichment and isolation of nitropropanol-metabolizing bacterium from the rumen. Appl. Environ. Microbiol. 1996, 62, 3885–3886. [Google Scholar]
- Anderson, R.C.; Rasmussen, M.A.; Dispirito, A.A.; Allison, M.J. Characteristics of a nitropropanol-metabolizing bacterium isolated from the rumen. Can. J. Microbiol. 1997, 43, 617–624. [Google Scholar] [CrossRef]
- Anderson, R.C.; Rasmussen, M.A.; Jensen, N.S.; Allison, M.J. Denitrobacterium detoxificans gen. nov., sp. nov., a ruminal bacterium that respires on nitrocompounds. Int. J. Syst. Evol. Microbiol. 2000, 50, 633–638. [Google Scholar] [CrossRef] [Green Version]
- Cheng, K.J.; Phillippe, R.C.; Kozub, G.C.; Majak, W.; Costerton, J.W. Induction of nitrite and nitrite metabolism in bovine rumen fluid and the transfer of this capacity to untreated animals. Can. Vet. J. 1985, 65, 647–652. [Google Scholar] [CrossRef]
- Soltan, Y.A.; Hashem, N.M.; Morsy, A.S.; El-Azrak, K.M.; Sallam, S.M. Comparative effects of Moringa oleifera root bark and monensin supplementations on ruminal fermentation, nutrient digestibility and growth performance of growing lambs. Anim. Feed Sci. Technol. 2018, 235, 189–201. [Google Scholar] [CrossRef]
- Anderson, R.C.; Krueger, N.A.; Stanton, T.B.; Callaway, T.R.; Edrington, T.S.; Harvey, R.B.; Jung, Y.S.; Nisbet, D.J. Effects of select nitrocompounds on in vitro ruminal fermentation during conditions of limiting or excess added reductant. Bioresour. Technol. 2008, 99, 8655–8661. [Google Scholar] [CrossRef] [PubMed]
- Scott, E.W. The metabolism of nitroparaffins. II. The metabolic products of nitroethane. J. Ind. Hyg. Toxicol. 1942, 24, 226–228. [Google Scholar]
- Angermaier, L.; Simon, H. On the reduction of aliphatic and aromatic nitro compounds by Clostridia, the role of ferredoxin and its stabilization. Hoppe Seylers. Z. Physiol. Chem. 1983, 364, 961–975. [Google Scholar] [CrossRef] [PubMed]
- Latham, E.A.; Anderson, R.C.; Pinchak, W.E.; Nisbet, D.J. Insights on alterations to the rumen ecosystem by nitrate and nitrocompounds. Front. Microbiol. 2016, 7, 228. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.J.; Rooke, J.A.; Cabeza, I.; Wallace, R.J. Nitrate and inhibition of ruminal methanogenesis: Microbial ecology, obstacles, and opportunities for lowering methane emissions from ruminant livestock. Front. Microbiol. 2016, 7, 132. [Google Scholar] [CrossRef]
- Bradley, W.B.; Eppson, H.F.; Beath, O.A. Nitrate as the cause of oat hay poisoning. J. Am. Vet. Med. Assoc. 1939, 94, 541–546. [Google Scholar]
- Menke, K.H.; Steingass, H. Estimation of the energetic feed value obtained by chemical analysis and in vitro gas production using rumen fluid. Anim. Res. Dev. 1988, 28, 7–55. [Google Scholar]
- Deng, T.; Hu, S.; Huang, X.A.; Song, J.; Xu, Q.; Wang, Y.; Liu, F. A novel strategy for colorimetric detection of hydroxyl radicals based on a modified Griess test. Talanta 2019, 195, 152–157. [Google Scholar] [CrossRef]
- Pang, D.G.; Yang, H.J.; Cao, B.B.; Wu, T.T.; Wang, J.Q. The beneficial effect of Enterococcus faecium on the in vitro ruminal fermentation rate and extent of three typical total mixed rations in northern China. Livest. Sci. 2014, 167, 154–160. [Google Scholar] [CrossRef]
- Bai, S.; Cao, Z.J.; Cao, B.B.; Yang, H.J.; Li, S.L.; Liu, J.X. Effects of different forage combinations in total mixed rations on in vitro gas production kinetics, ruminal and milk fatty acid profiles of lactating cows. Anim. Sci. J. 2018, 89, 1261–1270. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.F.; Yang, H.J. In vitro ruminal methanogenesis of a hay-rich substrate in response to different combination supplements of nitrocompounds; pyromellitic diimide and 2-bromoethanesulphonate. Anim. Feed Sci. Technol. 2011, 163, 20–32. [Google Scholar] [CrossRef]
- Dhillon, S.; Gill, K. Clinical Pharmacokinetics. In Basic Pharmacokinetics; Pharmaceutical Press: London, UK, 2006. [Google Scholar]

| Nitrocompounds 1 | p-Value 2 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Items 3 | Substrate | CTR | NE | NEOH | NPOH | SEM | S | N | S × N |
| total VFA, mmol | HF | 6.8 | 6.7 | 6.6 | 6.8 | 0.15 | < 0.01 | 0.63 | 0.79 |
| LF | 7.5 | 7.4 | 7.2 | 7.2 | |||||
| Molar percentage of individual VFA, mol/100 mol | |||||||||
| Acetate | HF | 60.8 a | 59.7 b | 59.3 b | 59.2 b | 0.41 | < 0.01 | < 0.01 | 0.17 |
| LF | 57.1 a | 54.1 b | 54.0 b | 54.1 b | |||||
| Propionate | HF | 23.0 c | 23.5 b | 24.2 a | 24.0 a | 0.18 | < 0.01 | < 0.01 | 0.07 |
| LF | 24.6 c | 26.0 b | 27.3 a | 26.4 b | |||||
| Butyrate | HF | 9.4 b | 10.4 a | 10.1 a | 10.1 a | 0.12 | < 0.01 | < 0.01 | 0.13 |
| LF | 11.7 b | 12.7 a | 12.4 a | 12.0 a | |||||
| Valerate | HF | 1.6 | 1.5 | 1.5 | 1.6 | 0.03 | 0.21 | 0.10 | 0.56 |
| LF | 1.6 | 1.5 | 1.5 | 1.5 | |||||
| BCVFA | HF | 4.8 | 5.0 | 5.1 | 4.8 | 0.27 | < 0.01 | 0.33 | 0.61 |
| LF | 4.9 | 6.2 | 5.5 | 5.7 | |||||
| Ace:Pro | HF | 2.7 a | 2.6 b | 2.4 c | 2.5 bc | 0.03 | < 0.01 | < 0.01 | 0.15 |
| LF | 2.3 a | 2.1 b | 2.0 b | 2.1 b | |||||
| FE | HF | 75.0 c | 75.6 b | 76.0 a | 75.8 ab | 0.13 | < 0.01 | < 0.01 | 0.08 |
| LF | 76.4 b | 77.4 a | 77.7 a | 77.5 a | |||||
| Nitrocompounds 1 | p-Value 2 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Items 3 | Substrate | CTR | NE | NEOH | NPOH | SEM | S | N | S × N |
| GP120, mL/kg DM | HF | 147 a | 128 b | 128 b | 145 a | 3.3 | < 0.01 | < 0.01 | 0.11 |
| - | LF | 217 a | 183 bc | 172 c | 187 b | - | - | - | - |
| CH4, % | HF | 21.0 a | 0.3 c | 0.5 c | 13.4 b | 0.05 | < 0.01 | < 0.01 | 0.23 |
| - | LF | 21.1 a | 0.2 c | 0.2 c | 8.6 b | - | - | - | - |
| H2, % | HF | 0.1 d | 5.8 a | 3.7 b | 1.2 c | 0.05 | < 0.01 | < 0.01 | 0.09 |
| - | LF | 0.1 c | 9.6 a | 8.8 ab | 6.9 b | - | - | - | - |
| CO2, % | HF | 78.9 c | 93.8 a | 95.8 a | 85.4 b | 0.79 | 0.01 | < 0.01 | 0.10 |
| - | LF | 78.8 c | 90.3 a | 91.0 a | 84.5 b | - | - | - | - |
| Treatment 1 | p-Value 2 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Items 3 | Substrate | CTR | NE | NEOH | NPOH | SEM | S | N | S × N |
| C0, mM | HF | 0 | 0.75 | 0.75 | 0.75 | - | - | - | - |
| LF | 0 | 0.75 | 0.75 | 0.75 | - | - | - | - | |
| k, %/h | HF | 0 | 2.8 a | 1.1 b | 0.8 b | 0.42 | < 0.01 | < 0.01 | 0.11 |
| LF | 0 | 6.2 a | 1.2 b | 0.6 b | - | - | - | - | |
| T1/2, h | HF | 0 | 4.6 c | 5.3 b | 5.5 a | 0.08 | < 0.01 | < 0.01 | 0.12 |
| LF | 0 | 3.7 c | 5.1 b | 5.7 a | - | - | - | - | |
| Treatment 1 | p-Value 2 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Items 3 | Substrate | CTR | NE | NEOH | NPOH | SEM | S | N | S × N |
| C3, mM | HF | 0 | 0.11 c | 0.40 a | 0.17 b | 0.012 | < 0.01 | < 0.01 | 0.13 |
| LF | 0 | 0.09 c | 0.26 a | 0.15 b | |||||
| k, %/h | HF | 0 | 1.2 a | 1.0 b | 1.0 b | 0.02 | 0.83 | < 0.01 | 0.12 |
| LF | 0 | 1.1 a | 1.1 b | 0.9 b | |||||
| T1/2, h | HF | 0 | 5.1 b | 5.3 a | 5.3 a | 0.01 | 0.47 | < 0.01 | 0.21 |
| LF | 0 | 5.2 b | 5.2 b | 5.4 a | |||||
| Treatment 1 | p-Value 2 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Items 3 | Substrate | CTR | NE | NEOH | NPOH | SEM | S | N | S × N |
| A, mM | HF | 2.5 a | 2.3 b | 1.9 c | 2.4 a | 0.11 | 0.01 | 0.01 | 0.54 |
| LF | 2.4 a | 1.8 b | 1.4 c | 1.7 b | |||||
| k, %/h | HF | 1.1 a | 1.0 a | 0.9 b | 1.0 a | 0.01 | < 0.01 | < 0.01 | 0.09 |
| LF | 1.0 a | 0.8 b | 0.6 c | 0.7 b | |||||
| T1/2, h | HF | 5.2 c | 5.3 b | 5.5 a | 5.3 b | 0.02 | < 0.01 | < 0.01 | 0.12 |
| LF | 5.3 c | 5.5 b | 5.8 a | 5.6 b | |||||
| Treatment 1 | p-Value 2 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Items 3 | Substrate | CTR | NE | NEOH | NPOH | SEM | S | N | S × N |
| A, g/L | HF | 1.8 b | 2.2 a | 2.1 a | 2.2 a | 0.13 | 0.96 | 0.03 | 0.89 |
| LF | 1.8 b | 2.3 a | 2.1 a | 2.1 a | |||||
| k, %/h | HF | 0.6 b | 0.8 a | 0.7 a | 0.8 a | 0.02 | 0.41 | 0.02 | 0.12 |
| LF | 0.6 b | 0.8 a | 0.8 a | 0.8 a | |||||
| T1/2, h | HF | 5.8 a | 5.5 b | 5.6 b | 5.5 b | 0.02 | 0.09 | < 0.01 | 0.11 |
| LF | 5.7 a | 5.5 b | 5.6 b | 5.5 b | |||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.-W.; Wang, Y.-L.; Wang, W.-K.; Chen, Y.-Y.; Si, X.-M.; Wang, Y.-J.; Wang, W.; Cao, Z.-J.; Li, S.-L.; Yang, H.-J. The Antimethanogenic Nitrocompounds Can be Cleaved into Nitrite by Rumen Microorganisms: A Comparison of Nitroethane, 2-Nitroethanol, and 2-Nitro-1-propanol. Metabolites 2020, 10, 15. https://doi.org/10.3390/metabo10010015
Zhang Z-W, Wang Y-L, Wang W-K, Chen Y-Y, Si X-M, Wang Y-J, Wang W, Cao Z-J, Li S-L, Yang H-J. The Antimethanogenic Nitrocompounds Can be Cleaved into Nitrite by Rumen Microorganisms: A Comparison of Nitroethane, 2-Nitroethanol, and 2-Nitro-1-propanol. Metabolites. 2020; 10(1):15. https://doi.org/10.3390/metabo10010015
Chicago/Turabian StyleZhang, Zhen-Wei, Yan-Lu Wang, Wei-Kang Wang, Yong-Yang Chen, Xue-Meng Si, Ya-Jing Wang, Wei Wang, Zhi-Jun Cao, Sheng-Li Li, and Hong-Jian Yang. 2020. "The Antimethanogenic Nitrocompounds Can be Cleaved into Nitrite by Rumen Microorganisms: A Comparison of Nitroethane, 2-Nitroethanol, and 2-Nitro-1-propanol" Metabolites 10, no. 1: 15. https://doi.org/10.3390/metabo10010015