Homogenization and Growth Behavior of Second-Phase Particles in a Deformed Zr–Sn–Nb–Fe–Cu–Si–O Alloy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material Preparation
2.2. Characterizations
2.3. Corrosion Tests
3. Results and Discussion
3.1. Microstructure of the β-Quenched Sample
3.2. Microstructures of the Rolled Samples
3.3. Distribution and Growth Behavior of Second-Phase Particles after Heat Treatment
3.4. Mechanism for the Homogenization and Growth Behavior of Second-Phase Particles
3.5. Corrosion Resistance
5. Conclusions
- (1)
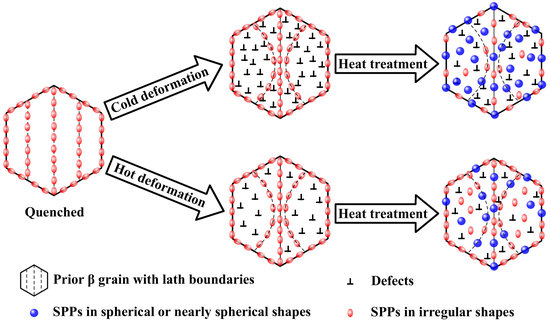
- A linear distribution of SPPs is presented in the quenched sample. After rolling, the distributions of SPPs in the samples are distorted to some extent, according to the deformation degree. The SPPs both in rolled samples are coarsened, spherized, and homogeneously distributed during annealing; the average diameters of the SPPs increase with increasing the deformation degree and annealing time (or temperature). Furthermore, compared with the cold-rolled–annealed samples, the emergences of the homogeneous distributions of the SPPs in the hot-rolled–annealed samples are postponed.
- (2)
- The homogenization and growth of SPPs are attributed to the Ostwald ripening mechanism. Due to the invariable volume fractions of SPPs in quenched, rolled, and annealed samples, the growth rates of SPPs are primarily governed by the diffusion rates of the substance of SPPs in the Zr matrix. Since the effective diffusion coefficient consists of the lattice diffusion and short-circuit diffusion, the sample with a higher deformation degree is speculated to have more defects in the Zr matrix, which provides more shortcuts for the mass transfer of SPPs. As a result, SPPs in the sample with a higher deformation degree grow and homogenize more easily compared with those in the sample with a lower deformation degree.
- (3)
- All the selected samples had a similar weight gain before the 100-day exposure. However, the samples with inhomogeneously distributed SPPs had a post-transition phenomenon after the 100-day exposure, whereas the transitions in corrosion kinetics of the samples with homogeneously distributed SPPs took place after the 160-day exposure. Therefore, it confirms a detrimental effect of inhomogeneously distributed SPPs on the corrosion resistance of the Zr alloys.
Author Contributions
Funding
Conflicts of Interest
References
- Chai, L.; Wang, T.; Ren, Y.; Song, B.; Guo, N.; Chen, L. Microstructural and Textural Differences Induced by Water and Furnace Cooling in Commercially Pure Zr Annealed in the α + β Region. Met. Mater. Int. 2018, 24, 673–680. [Google Scholar] [CrossRef]
- Chen, L.Y.; Shen, P.; Zhang, L.; Lu, S.; Chai, L.; Yang, Z.; Zhang, L.C. Corrosion behavior of non-equilibrium Zr-Sn-Nb-Fe-Cu-O alloys in high-temperature 0.01 M LiOH aqueous solution and degradation of the surface oxide films. Corros. Sci. 2018, 136, 221–230. [Google Scholar] [CrossRef]
- Chai, L.; Wu, H.; Wang, S.; Chen, K.; Wang, T.; Xia, J. Characterization of microstructure and hardness of a Zr-2.5Nb alloy surface-treated by pulsed laser. Mater. Chem. Phys. 2017, 198, 303–309. [Google Scholar] [CrossRef]
- Chai, L.J.; Wang, S.Y.; Wu, H.; Guo, N.; Pan, H.C.; Chen, L.Y.; Murty, K.L.; Song, B. α→β Transformation characteristics revealed by pulsed laser-induced non-equilibrium microstructures in duplex-phase Zr alloy. Sci. China Technol. Sci. 2017, 60, 1255–1262. [Google Scholar] [CrossRef]
- Zhang, M.; Li, Y.N.; Zhang, F.C.; Wang, X.B.; Chen, L.Y.; Yang, Z.N. Effect of annealing treatment on the microstructure and mechanical properties of a duplex Zr-2.5 Nb alloy. Mater. Sci. Eng. A 2017, 706, 236–241. [Google Scholar] [CrossRef]
- Yang, Z.N.; Zhang, F.C.; Qu, L.; Yan, Z.G.; Xiao, Y.Y.; Liu, R.P.; Zhang, X.Y. Formation of duplex microstructure in Zr-2.3Nb alloy and its plastic behaviour at various strain rates. Int. J. Plast. 2014, 54, 163–177. [Google Scholar] [CrossRef]
- Tao, B.; Qiu, R.; Zhao, Y.; Liu, Y.; Tan, X.; Luan, B.; Liu, Q. Effects of alloying elements (Sn, Cr and Cu) on second phase particles in Zr-Sn-Nb-Fe-(Cr, Cu) alloys. J. Alloys Compd. 2018, 748, 745–757. [Google Scholar] [CrossRef]
- Chen, L.; Li, J.; Zhang, Y.; Zhang, L.C.; Lu, W.; Wang, L.; Zhang, L.; Zhang, D. Zr-Sn-Nb-Fe-Si-O alloy for fuel cladding candidate: Processing, microstructure, corrosion resistance and tensile behavior. Corros. Sci. 2015, 100, 332–340. [Google Scholar] [CrossRef]
- Yang, H.; Shen, J.; Matsukawa, Y.; Satoh, Y.; Kano, S.; Zhao, Z.; Li, Y.; Li, F.; Abe, H. Effects of alloying elements (Sn, Nb, Cr, and Mo) on the microstructure and mechanical properties of zirconium alloys. J. Nucl. Sci. Technol. 2015, 52, 1162–1173. [Google Scholar] [CrossRef]
- Chen, L.; Zeng, Q.; Li, J.; Lu, J.; Zhang, Y.; Zhang, L.-C.; Qin, X.; Lu, W.; Zhang, L.; Wang, L.; et al. Effect of microstructure on corrosion behavior of a Zr–Sn–Nb–Fe–Cu–O alloy. Mater. Des. 2016, 92, 888–896. [Google Scholar] [CrossRef] [Green Version]
- Granovsky, M.S.; Canay, M.; Lena, E.; Arias, D. Experimental investigation of the Zr corner of the ternary Zr–Nb–Fe phase diagram. J. Nucl. Mater. 2002, 302, 1–8. [Google Scholar] [CrossRef]
- Yang, H.L.; Shen, J.J.; Kano, S.; Matsukawa, Y.; Li, Y.F.; Satoh, Y.; Matsunaga, T.; Abe, H. Effects of Mo addition on precipitation in Zr-1.2Nb alloys. Mater. Lett. 2015, 158, 88–91. [Google Scholar] [CrossRef]
- Toffolon-Masclet, C.; Guilbert, T.; Brachet, J.C. Study of secondary intermetallic phase precipitation/dissolution in Zr alloys by high temperature-high sensitivity calorimetry. J. Nucl. Mater. 2008, 372, 367–378. [Google Scholar] [CrossRef]
- Chen, L.; Li, J.; Zhang, Y.; Zhang, L.C.; Lu, W.; Zhang, L.; Wang, L.; Zhang, D. Effects of alloyed Si on the autoclave corrosion performance and periodic corrosion kinetics in Zr-Sn-Nb-Fe-O alloys. Corros. Sci. 2015, 100, 651–662. [Google Scholar] [CrossRef]
- Kudiiarov, N.V.; Larionov, V.V.; Tyurin, I.Y. Mechanical Property Testing of Hydrogenated Zirconium Irradiated with Electrons. Metals 2018, 8, 207. [Google Scholar] [CrossRef]
- Obrosov, A.; Sutygina, N.A.; Manakhov, A.; Bolz, S.; Weiß, S.; Kashkarov, B.E. Oxidation Behavior of Zr–1Nb Corroded in Air at 400 °C after Plasma Immersion Titanium Implantation. Metals 2018, 8, 27. [Google Scholar] [CrossRef]
- Priamushko, T.; Mikhaylov, A.; Babikhina, M.; Kudiiarov, V.; Laptev, R. Glow Discharge Optical Emission Spectrometer Calibration Using Hydrogenated Zr-2.5Nb Alloy Standard Samples. Metals 2018, 8, 372. [Google Scholar] [CrossRef]
- Proff, C.; Abolhassani, S.; Lemaignan, C. Oxidation behaviour of zirconium alloys and their precipitates—A mechanistic study. J. Nucl. Mater. 2013, 432, 222–238. [Google Scholar] [CrossRef]
- Kim, H.G.; Park, J.Y.; Jeong, Y.H. Ex-reactor corrosion and oxide characteristics of Zr-Nb-Fe alloys with the Nb/Fe ratio. J. Nucl. Mater. 2005, 345, 1–10. [Google Scholar] [CrossRef]
- Huang, J.; Yao, M.Y.; Gao, C.Y.; Liang, X.; Peng, J.C.; Zhang, J.L.; Zhou, B.X. The influence of second phase particles on the crack formation in oxide films formed on zirconium alloys. Corros. Sci. 2015, 99, 172–177. [Google Scholar] [CrossRef]
- Abe, H.; Iwamoto, T.; Yamamoto, Y.; Nishida, S.; Komatsu, R. Dimensional accuracy of tubes in cold pilgering. J. Mater. Process. Technol. 2016, 231, 277–287. [Google Scholar] [CrossRef]
- Chen, L.; Li, J.; Zhang, Y.; Lu, W.; Zhang, L.C.; Wang, L.; Zhang, D. Effect of low-temperature pre-deformation on precipitation behavior and microstructure of a Zr-Sn-Nb-Fe-Cu-O alloy during fabrication. J. Nucl. Sci. Technol. 2016, 53, 496–507. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, F.; Yang, Z.; Li, Y.; Qu, L.; Zhen, H. Effect of cooling process on the formation of duplex microstructure in Zr–2.3Nb alloy. J. Alloys Compd. 2015, 651, 316–321. [Google Scholar] [CrossRef]
- Yang, H.L.; Matsukawa, Y.; Kano, S.; Duan, Z.G.; Murakami, K.; Abe, H. Investigation on microstructural evolution and hardening mechanism in dilute Zr-Nb binary alloys. J. Nucl. Mater. 2016, 481, 117–124. [Google Scholar] [CrossRef]
- Chai, L.; Luan, B.; Murty, K.L.; Liu, Q. Effect of predeformation on microstructural evolution of a Zr alloy during 550-700 °C aging after β-quenching. Acta Mater. 2013, 61, 3099–3109. [Google Scholar] [CrossRef]
- Gros, J.P.; Wadier, J.F. Precipitate growth kinetics in Zircaloy-4. J. Nucl. Mater. 1990, 172, 85–96. [Google Scholar] [CrossRef]
- Liu, Y.; Kathan, K.; Saad, W.; Prud, R.K. Ostwald ripening of β-carotene nanoparticles. Phys. Rev. Lett. 2007, 98, 8–11. [Google Scholar] [CrossRef] [PubMed]
- Luan, B.F.; Chai, L.J.; Chen, J.W.; Zhang, M.; Liu, Q. Growth behavior study of second phase particles in a Zr-Sn-Nb-Fe-Cr-Cu alloy. J. Nucl. Mater. 2012, 423, 127–131. [Google Scholar] [CrossRef]
- Zhang, L.C.; Attar, H. Selective Laser Melting of Titanium Alloys and Titanium Matrix Composites for Biomedical Applications: A Review. Adv. Eng. Mater. 2016, 18, 463–475. [Google Scholar] [CrossRef]
- Calin, M.; Zhang, L.C.; Eckert, J. Tailoring of microstructure and mechanical properties of a Ti-based bulk metallic glass-forming alloy. Scr. Mater. 2007, 57, 1101–1104. [Google Scholar] [CrossRef]
- Chai, L.; Chen, K.; Zhi, Y.; Murty, K.L.; Chen, L.Y.; Yang, Z. Nanotwins induced by pulsed laser and their hardening effect in a Zr alloy. J. Alloys Compd. 2018, 748, 163–170. [Google Scholar] [CrossRef]
- Liu, L.H.; Yang, C.; Wang, F.; Qu, S.G.; Li, X.Q.; Zhang, W.W.; Li, Y.Y.; Zhang, L.C. Ultrafine grained Ti-based composites with ultrahigh strength and ductility achieved by equiaxing microstructure. Mater. Des. 2015, 79, 1–5. [Google Scholar] [CrossRef]
- Lumley, S.C.; Murphy, S.T.; Burr, P.A.; Grimes, R.W.; Chard-Tuckey, P.R.; Wenman, M.R. The stability of alloying additions in Zirconium. J. Nucl. Mater. 2013, 437, 122–129. [Google Scholar] [CrossRef]
- Herb, B.; Ruhrmann, H.; Konig, A. In-process investigation of precipitate growth in zirconium alloys. In Zirconium in the Nuclear Industry: Twelfth International Symposium; ASTM International: West Conshohocken, PA, USA, 2000; pp. 482–504. [Google Scholar]
- Chai, L.J.; Luan, B.F.; Gao, S.S.; Chen, J.W.; Liu, Q. Study of precipitate evolution and recrystallization of β-quenched Zr-Sn-Nb-Fe-Cr-Cu alloy during aging. J. Nucl. Mater. 2012, 427, 274–281. [Google Scholar] [CrossRef]
- Zhang, L.C.; Shen, Z.Q.; Xu, J. Mechanically milling-induced amorphization in Sn-containing Ti-based multicomponent alloy systems. Mater. Sci. Eng. A 2005, 394, 204–209. [Google Scholar] [CrossRef]
- Yu, P.; Zhang, L.C.; Zhang, W.Y.; Das, J.; Kim, K.B.; Eckert, J. Interfacial reaction during the fabrication of Ni60Nb40 metallic glass particles-reinforced Al based MMCs. Mater. Sci. Eng. A 2007, 444, 206–213. [Google Scholar] [CrossRef]
- Zhang, L.C.; Xu, J.; Ma, E. Mechanically Alloyed Amorphous Ti50(Cu0.45Ni0.55)44–xAlxSi4B2 Alloys with Supercooled Liquid Region. J. Mater. Res. 2002, 17, 1743–1749. [Google Scholar] [CrossRef]
- Zhang, L.C.; Kim, K.B.; Yu, P.; Zhang, W.Y.; Kunz, U.; Eckert, J. Amorphization in mechanically alloyed (Ti, Zr, Nb)-(Cu, Ni)-Al equiatomic alloys. J. Alloys Compd. 2007, 428, 157–163. [Google Scholar] [CrossRef]
- Jiang, L.; Pérez-Prado, M.T.; Gruber, P.A.; Arzt, E.; Ruano, O.A.; Kassner, M.E. Texture, microstructure and mechanical properties of equiaxed ultrafine-grained Zr fabricated by accumulative roll bonding. Acta Mater. 2008, 56, 1228–1242. [Google Scholar] [CrossRef]
- Zhu, K.Y.; Chaubet, D.; Bacroix, B.; Brisset, F. A study of recovery and primary recrystallization mechanisms in a Zr-2Hf alloy. Acta Mater. 2005, 53, 5131–5140. [Google Scholar] [CrossRef]
- Kahlweit, M. Ostwald ripening of precipitates. Adv. Colloid Interface Sci. 1975, 5, 1–35. [Google Scholar] [CrossRef]
- Yang, H.L.; Kano, S.; Matsukawa, Y.; Li, Y.F.; Shen, J.J.; Li, F.; Zhao, Z.S.; Satoh, Y.; Abe, H. Effect of molybdenum on microstructures in Zr-1.2Nb alloys after β-quenching and subsequently 873 K annealing. Mater. Des. 2016, 104, 355–364. [Google Scholar] [CrossRef]
- Zhang, L.C.; Liu, Y.; Li, S.; Hao, Y. Additive Manufacturing of Titanium Alloys by Electron Beam Melting: A Review. Adv. Eng. Mater. 2018, 20, 1700842. [Google Scholar] [CrossRef]
- Marqusee, J.A.; Ross, J. Theory of Ostwald ripening: Competitive growth and its dependence on volume fraction. J. Chem. Phys. 1984, 80, 536–543. [Google Scholar] [CrossRef]
- Lifshitz, I.M.; Slyozov, V.V. The kinetics of precipitation from supersaturated solid solutions. J. Phys. Chem. Solids 1961, 19, 35–50. [Google Scholar] [CrossRef]
- Carman, A.; Zhang, L.C.; Ivasishin, O.M.; Savvakin, D.G.; Matviychuk, M.V.; Pereloma, E.V. Role of alloying elements in microstructure evolution and alloying elements behaviour during sintering of a near-β titanium alloy. Mater. Sci. Eng. A 2011, 528, 1686–1693. [Google Scholar] [CrossRef]
- Sun, C.Y.; Cong, Y.P.; Zhang, Q.D.; Fu, M.W.; Li, L. Element diffusion model with variable coefficient in bimetallic bonding process. J. Mater. Process. Technol. 2018, 253, 99–108. [Google Scholar] [CrossRef]
- Qin, P.; Liu, Y.; Sercombe, T.B.; Li, Y.; Zhang, C.; Cao, C.; Sun, H.; Zhang, L.C. Improved Corrosion Resistance on Selective Laser Melting Produced Ti-5Cu Alloy after Heat Treatment. ACS Biomater. Sci. Eng. 2018, 4, 2633–2642. [Google Scholar] [CrossRef]
- Qin, X.; Guo, X.; Lu, J.; Chen, L.; Qin, J.; Lu, W. Erosion-wear and intergranular corrosion resistance properties of AISI 304L austenitic stainless steel after low-temperature plasma nitriding. J. Alloys Compd. 2017, 698, 1094–1101. [Google Scholar] [CrossRef]
- Panicaud, B.; Grosseau-Poussard, J.L.; Retraint, D.; Guérain, M.; Li, L. On the mechanical effects of a nanocrystallisation treatment for ZrO2 oxide films growing on a zirconium alloy. Corros. Sci. 2013, 68, 263–274. [Google Scholar] [CrossRef]
- Zhang, L.C.; Lu, H.B.; Mickel, C.; Eckert, J. Ductile ultrafine-grained Ti-based alloys with high yield strength. Appl. Phys. Lett. 2007, 91, 051906. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Li, G.P.; Wang, H.; Wu, S.Q.; Zhang, L.C.; Li, Y.L.; Yang, K. Formation of zigzag-shaped {1 1 2}<1 1 1> β mechanical twins in Ti-24.5 Nb-0.7 Ta-2 Zr-1.4 O alloy. Scr. Mater. 2012, 66, 211–214. [Google Scholar] [CrossRef]
- Wang, L.; Xie, L.; Lv, Y.; Zhang, L.C.; Chen, L.; Meng, Q.; Qu, J.; Zhang, D.; Lu, W. Microstructure evolution and superelastic behavior in Ti-35Nb-2Ta-3Zr alloy processed by friction stir processing. Acta Mater. 2017, 131, 499–510. [Google Scholar] [CrossRef]
- Proff, C.; Abolhassani, S.; Lemaignan, C. Oxidation behaviour of binary zirconium alloys and their precipitates. J. Nucl. Mater. 2013, 432, 1–22. [Google Scholar] [CrossRef]










© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, L.-Y.; Sang, P.; Zhang, L.; Song, D.; Chu, Y.-Q.; Chai, L.; Zhang, L.-C. Homogenization and Growth Behavior of Second-Phase Particles in a Deformed Zr–Sn–Nb–Fe–Cu–Si–O Alloy. Metals 2018, 8, 759. https://doi.org/10.3390/met8100759
Chen L-Y, Sang P, Zhang L, Song D, Chu Y-Q, Chai L, Zhang L-C. Homogenization and Growth Behavior of Second-Phase Particles in a Deformed Zr–Sn–Nb–Fe–Cu–Si–O Alloy. Metals. 2018; 8(10):759. https://doi.org/10.3390/met8100759
Chicago/Turabian StyleChen, Liang-Yu, Peng Sang, Lina Zhang, Dongpo Song, Yan-Qiu Chu, Linjiang Chai, and Lai-Chang Zhang. 2018. "Homogenization and Growth Behavior of Second-Phase Particles in a Deformed Zr–Sn–Nb–Fe–Cu–Si–O Alloy" Metals 8, no. 10: 759. https://doi.org/10.3390/met8100759