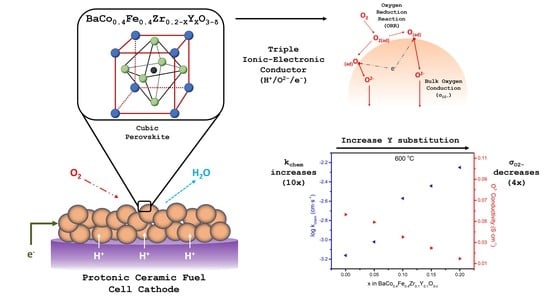
Surface and Bulk Oxygen Kinetics of BaCo0.4Fe0.4Zr0.2−XYXO3−δ Triple Conducting Electrode Materials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of BCFZYX Precursor Powders
2.2. Fabrication of Dense BCFZYX Pellets for Characterization
2.3. Characterization
3. Results
3.1. Structure and Composition
3.2. Oxygen Permeation Properties
3.3. Conductivity
3.4. Electrical Conductivity Relaxation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Duan, C.; Tong, J.; Shang, M.; Nikodemski, S.; Sanders, M.; Ricote, S.; Almansoori, A.; OHayre, R. Readily processed protonic ceramic fuel cells with high performance at low temperatures. Science 2015, 349, 1321–1326. [Google Scholar] [CrossRef]
- Xu, D.; Dong, F.; Chen, Y.; Zhao, B.; Liu, S.; Tade, M.O.; Shao, Z. Cobalt-free niobium-doped barium ferrite as potential materials of dense ceramic membranes for oxygen separation. J. Memb. Sci. 2014, 455, 75–82. [Google Scholar] [CrossRef]
- Tomura, Y.; Tazawa, T.; Oikawa, I.; Takamura, H. Catalytic activity for dissociative oxygen adsorption of Co-based oxides at high temperature evaluated by a modified pulse isotopic exchange technique. J. Mater. Chem. A 2020, 8, 21634–21641. [Google Scholar] [CrossRef]
- Zohourian, R.; Merkle, R.; Raimondi, G.; Maier, J. Mixed-Conducting Perovskites as Cathode Materials for Protonic Ceramic Fuel Cells: Understanding the Trends in Proton Uptake. Adv. Funct. Mater. 2018, 28, 1801241. [Google Scholar] [CrossRef]
- Choi, S.; Kucharczyk, C.J.; Liang, Y.; Zhang, X.; Takeuchi, I.; Ji, H.-I.; Haile, S.M. Exceptional power density and stability at intermediate temperatures in protonic ceramic fuel cells. Nat. Energy 2018, 3, 202–210. [Google Scholar] [CrossRef] [Green Version]
- Papac, M.; Stevanović, V.; Zakutayev, A.; O’Hayre, R. Triple ionic–electronic conducting oxides for next-generation electrochemical devices. Nat. Mater. 2021, 20, 301–313. [Google Scholar] [CrossRef] [PubMed]
- Seong, A.; Kim, J.; Kim, J.; Kim, S.; Sengodan, S.; Shin, J.; Kim, G. Influence of Cathode Porosity on High Performance Protonic Ceramic Fuel Cells with PrBa0.5Sr0.5Co1.5Fe0.5O5-δ Cathode. J. Electrochem. Soc. 2018, 165, F1098–F1102. [Google Scholar] [CrossRef]
- Endler-Schuck, C.; Joos, J.; Niedrig, C.; Weber, A.; Ivers-Tiffée, E. The chemical oxygen surface exchange and bulk diffusion coefficient determined by impedance spectroscopy of porous La0.58Sr0.4Co0.2Fe0.8O3−δ (LSCF) cathodes. Solid State Ionics 2015, 269, 67–79. [Google Scholar] [CrossRef]
- Dierickx, S.; Weber, A.; Ivers-Tiffée, E. How the distribution of relaxation times enhances complex equivalent circuit models for fuel cells. Electrochim. Acta 2020, 355, 136764. [Google Scholar] [CrossRef]
- Wang, S.; Verma, A.; Yang, Y.L.; Jacobson, A.J.; Abeles, B. The effect of the magnitude of the oxygen partial pressure change in electrical conductivity relaxation measurements: Oxygen transport kinetics in La0.5Sr0.5CoO3−δ. Solid State Ionics 2001, 140, 125–133. [Google Scholar] [CrossRef]
- Li, Y.; Gerdes, K.; Diamond, H.; Liu, X. An improved method to increase the predictive accuracy of the ECR technique. Solid State Ionics 2011, 204–205, 104–110. [Google Scholar] [CrossRef]
- Li, Y.; Gerdes, K.; Liu, X. Oxygen Transport Kinetics in Infiltrated SOFCs Cathode by Electrical Conductivity Relaxation Technique. J. Electrochem. Soc. 2013, 160, F554–F559. [Google Scholar] [CrossRef]
- Li, Y.; Gerdes, K.; Horita, T.; Liu, X. Surface Exchange and Bulk Diffusivity of LSCF as SOFC Cathode: Electrical Conductivity Relaxation and Isotope Exchange Characterizations. J. Electrochem. Soc. 2013, 160, F343–F350. [Google Scholar] [CrossRef]
- Kim, G.; Wang, S.; Jacobson, A.J.; Chen, C.L. Measurement of oxygen transport kinetics in epitaxial La2NiO4+δ thin films by electrical conductivity relaxation. Solid State Ionics 2006, 177, 1461–1467. [Google Scholar] [CrossRef]
- Hong, T.; Lu, W.; Ren, K.; Liu, T. The two-fold diffusion process for proton uptake reaction in BCFZY e−/H+/O2− triple conductor measured by electrical conductivity relaxation. Ionics 2020, 26, 5293–5297. [Google Scholar] [CrossRef]
- Chen, Y.; Hong, T.; Wang, P.; Brinkman, K.; Tong, J.; Cheng, J. Investigate the proton uptake process of proton/oxygen ion/hole triple conductor BaCo0.4Fe0.4Zr0.1Y0.1O3−Δ by electrical conductivity relaxation. J. Power Sources 2019, 440, 227122. [Google Scholar] [CrossRef]
- Ren, R.; Wang, Z.; Meng, X.; Wang, X.; Xu, C.; Qiao, J.; Sun, W.; Sun, K. Tailoring the Oxygen Vacancy to Achieve Fast Intrinsic Proton Transport in a Perovskite Cathode for Protonic Ceramic Fuel Cells. ACS Appl. Energy Mater. 2020, 3, 4914–4922. [Google Scholar] [CrossRef]
- Ding, H.; Wu, W.; Jiang, C.; Ding, Y.; Bian, W.; Hu, B.; Singh, P.; Orme, C.J.; Wang, L.; Zhang, Y.; et al. Self-sustainable protonic ceramic electrochemical cells using a triple conducting electrode for hydrogen and power production. Nat. Commun. 2020, 11, 1907. [Google Scholar] [CrossRef]
- Choi, S.; Davenport, T.C.; Haile, S.M. Protonic ceramic electrochemical cells for hydrogen production and electricity generation: Exceptional reversibility, stability, and demonstrated faradaic efficiency. Energy Environ. Sci. 2019, 12, 206–215. [Google Scholar] [CrossRef]
- Wang, N.; Hinokuma, S.; Ina, T.; Zhu, C.; Habazaki, H.; Aoki, Y. Mixed proton-electron-oxide ion triple conducting manganite as an efficient cobalt-free cathode for protonic ceramic fuel cells. J. Mater. Chem. A 2020, 8, 11043–11055. [Google Scholar] [CrossRef]
- Chen, C.H.; Bouwmeester, H.J.M.; Van Doom, R.H.E.; Kruidhof, H.; Burggraaf, A.J. Oxygen permeation of La0.3Sr0.7CoO3−δ. Solid State Ionics 1997, 98, 7–13. [Google Scholar] [CrossRef]
- Balachandran, U.; Ma, B.; Maiya, P.S.; Mieville, R.L.; Dusek, J.T.; Picciolo, J.J.; Guan, J.; Dorris, S.E.; Liu, M. Development of mixed-conducting oxides for gas separation. Solid State Ionics 1998, 108, 363–370. [Google Scholar] [CrossRef] [Green Version]
- Balaguer, M.; Solís, C.; Serra, J.M. Study of the transport properties of the mixed ionic electronic conductor Ce1−xTbxO2−δ + Co (x = 0.1, 0.2) and evaluation as oxygen-transport membrane. Chem. Mater. 2011, 23, 2333–2343. [Google Scholar] [CrossRef] [Green Version]
- Zhou, C.; Sunarso, J.; Song, Y.; Dai, J.; Zhang, J.; Gu, B.; Zhou, W.; Shao, Z. New reduced-temperature ceramic fuel cells with dual-ion conducting electrolyte and triple-conducting double perovskite cathode. J. Mater. Chem. A 2019, 7, 13265–13274. [Google Scholar] [CrossRef]
- Meng, Y.; Duffy, J.; Na, B.T.; Gao, J.; Yang, T.; Tong, J.; Lee, S.; Brinkman, K.S. Oxygen exchange and bulk diffusivity of BaCo0.4Fe0.4Zr0.1Y0.1O3−δ: Quantitative assessment of active cathode material for protonic ceramic fuel cells. Solid State Ionics 2021, 368, 115639. [Google Scholar] [CrossRef]
- Ren, R.; Wang, Z.; Xu, C.; Sun, W.; Qiao, J.; Rooney, D.W.; Sun, K. Tuning the defects of the triple conducting oxide BaCo0.4Fe0.4Zr0.1Y0.1O3−δ perovskite toward enhanced cathode activity of protonic ceramic fuel cells. J. Mater. Chem. A 2019, 7, 18365–18372. [Google Scholar] [CrossRef]
- Li, X.; He, L.; Zhong, X.; Zhang, J.; Luo, S.; Yi, W.; Zhang, L.; Hu, M.; Tang, J.; Zhou, X.; et al. Evaluation of A-Site Ba2+-Deficient Ba1−xCo0.4Fe0.4Zr0.1Y0.1O3−δ Oxides as Electrocatalysts for Efficient Hydrogen Evolution Reaction. Scanning 2018, 2018, 1341608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, F.; Liang, M.; Wang, W.; Ran, R.; Yang, G.; Zhou, W.; Shao, Z. High-Performance Proton-Conducting Fuel Cell with B-Site-Deficient Perovskites for All Cell Components. Energy Fuels 2020, 34, 11464–11471. [Google Scholar] [CrossRef]
- Kuai, X.; Yang, G.; Chen, Y.; Sun, H.; Dai, J.; Song, Y.; Ran, R.; Wang, W.; Zhou, W.; Shao, Z. Boosting the Activity of BaCo0.4Fe0.4Zr0.1Y0.1O3−δ Perovskite for Oxygen Reduction Reactions at Low-to-Intermediate Temperatures through Tuning B-Site Cation Deficiency. Adv. Energy Mater. 2019, 9, 1902384. [Google Scholar] [CrossRef]
- Liang, M.; He, F.; Zhou, C.; Chen, Y.; Ran, R.; Yang, G.; Zhou, W.; Shao, Z. Nickel-doped BaCo0.4Fe0.4Zr0.1Y0.1O3−δ as a new high-performance cathode for both oxygen-ion and proton conducting fuel cells. Chem. Eng. J. 2021, 420, 127717. [Google Scholar] [CrossRef]
- Shang, M.; Tong, J.; O’Hayre, R. A promising cathode for intermediate temperature protonic ceramic fuel cells: BaCo0.4Fe0.4Zr0.2O3−δ. RSC Adv. 2013, 3, 15769–15775. [Google Scholar] [CrossRef]
- Duan, C.; Hook, D.; Chen, Y.; Tong, J.; O’Hayre, R. Zr and Y co-doped perovskite as a stable, high performance cathode for solid oxide fuel cells operating below 500 °C. Energy Environ. Sci. 2017, 10, 176–182. [Google Scholar] [CrossRef]
- Duan, C.; Huang, J.; Sullivan, N.; O’Hayre, R. Proton-conducting oxides for energy conversion and storage. Appl. Phys. Rev. 2020, 7, 011314. [Google Scholar] [CrossRef]
- Tong, J.; Yang, W.; Zhu, B.; Cai, R. Investigation of ideal zirconium-doped perovskite-type ceramic membrane materials for oxygen separation. J. Memb. Sci. 2002, 203, 175–189. [Google Scholar] [CrossRef]
- Mogensen, M.; Lybye, D.; Bonanos, N.; Hendriksen, P.V.; Poulsen, F.W. Factors controlling the oxide ion conductivity of fluorite and perovskite structured oxides. Solid State Ionics 2004, 174, 279–286. [Google Scholar] [CrossRef]
- Hong, W.K.; Choi, G.M. Oxygen permeation of BSCF membrane with varying thickness and surface coating. J. Memb. Sci. 2010, 346, 353–360. [Google Scholar] [CrossRef]
- Chen, D.; Shao, Z. Surface exchange and bulk diffusion properties of Ba0.5Sr0.5Co0.8Fe0.2O3−δ mixed conductor. Int. J. Hydrog. Energy 2011, 36, 6948–6956. [Google Scholar] [CrossRef]
- Wei, B.; Lü, Z.; Huang, X.; Miao, J.; Sha, X.; Xin, X.; Su, W. Crystal structure, thermal expansion and electrical conductivity of perovskite oxides BaxSr1−xCo0.8Fe0.2O3−δ (0.3 ≤ x ≤ 0.7). J. Eur. Ceram. Soc. 2006, 26, 2827–2832. [Google Scholar] [CrossRef]
- Jiang, L.; Wei, T.; Zeng, R.; Zhang, W.-X.; Huang, Y.-H. Thermal and electrochemical properties of PrBa0.5Sr0.5Co2−xFexO5+δ (x = 0.5, 1.0, 1.5) cathode materials for solid-oxide fuel cells. J. Power Sources 2013, 232, 279–285. [Google Scholar] [CrossRef]
- Poetzsch, D.; Merkle, R.; Maier, J. Proton uptake in the H + -SOFC cathode material Ba0.5Sr0.5Fe0.8Zn0.2O3−δ: Transition from hydration to hydrogenation with increasing oxygen partial pressure. Faraday Discuss. 2015, 182, 129–143. [Google Scholar] [CrossRef]
- Brinkman, K.; Iijima, T.; Takamura, H. The oxygen permeation characteristics of Bi1−xSrxFeO3 mixed ionic and electronic conducting ceramics. Solid State Ionics 2010, 181, 53–58. [Google Scholar] [CrossRef]
- Na, B.T.; Yang, T.; Liu, J.; Lee, S.; Abernathy, H.; Kalapos, T.; Hackett, G. Enhanced accuracy of electrochemical kinetic parameters determined by electrical conductivity relaxation. Solid State Ionics 2021, 361, 115561. [Google Scholar] [CrossRef]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duffy, J.H.; Meng, Y.; Abernathy, H.W.; Brinkman, K.S. Surface and Bulk Oxygen Kinetics of BaCo0.4Fe0.4Zr0.2−XYXO3−δ Triple Conducting Electrode Materials. Membranes 2021, 11, 766. https://doi.org/10.3390/membranes11100766
Duffy JH, Meng Y, Abernathy HW, Brinkman KS. Surface and Bulk Oxygen Kinetics of BaCo0.4Fe0.4Zr0.2−XYXO3−δ Triple Conducting Electrode Materials. Membranes. 2021; 11(10):766. https://doi.org/10.3390/membranes11100766
Chicago/Turabian StyleDuffy, Jack H., Yuqing Meng, Harry W. Abernathy, and Kyle S. Brinkman. 2021. "Surface and Bulk Oxygen Kinetics of BaCo0.4Fe0.4Zr0.2−XYXO3−δ Triple Conducting Electrode Materials" Membranes 11, no. 10: 766. https://doi.org/10.3390/membranes11100766