The Phase Structural Evolution and Gas Separation Performances of Cellulose Acetate/Polyimide Composite Membrane from Polymer to Carbon Stage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Membranes
2.3. Heat Treatment
2.4. Characterization
2.5. Permeation Measurements
3. Results
3.1. CA/PI Composite Membranes
3.2. Heat-Treated CA/PI Membranes
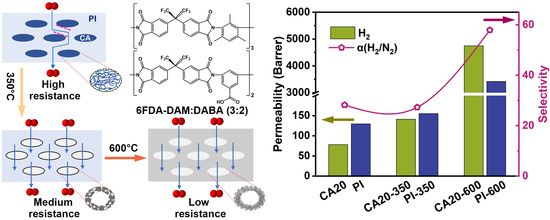
3.3. CA/PI-Based Carbon Molecule Sieve Membranes (CMSMs)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Salleh, W.N.W.; Ismail, A.F. Carbon membranes for gas separation processes: Recent progress and future perspective. J. Membr. Sci. Res. 2015, 1, 2–15. [Google Scholar]
- Saufi, S.M.; Ismail, A.F. Fabrication of carbon membranes for gas separation––A review. Carbon 2004, 42, 241–259. [Google Scholar] [CrossRef]
- Robeson, L.M. The upper bound revisited. J. Membr. Sci. 2008, 320, 390–400. [Google Scholar] [CrossRef]
- Robeson, L.M. Correlation of separation factor versus permeability for polymeric membranes. J. Membr. Sci. 1991, 62, 165–185. [Google Scholar] [CrossRef]
- Sanyal, O.; Zhang, C.; Wenz, G.B.; Fu, S.; Bhuwania, N.; Xu, L.; Rungta, M.; Koros, W.J. Next generation membranes—Using tailored carbon. Carbon 2018, 127, 688–698. [Google Scholar] [CrossRef]
- Koros, W.J.; Zhang, C. Materials for next-generation molecularly selective synthetic membranes. Nat. Mater. 2017, 16, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Llosa Tanco, M.A.; Pacheco Tanaka, D.A. Recent Advances on Carbon Molecular Sieve Membranes (CMSMs) and Reactors. Processes 2016, 4, 29. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Francisco, J.M.; Santiago-García, J.L.; Loría-Bastarrachea, M.I.; Paul, D.R.; Freeman, B.D.; Aguilar-Vega, M. CMS membranes from PBI/PI blends: Temperature effect on gas transport and separation performance. J. Membr. Sci. 2020, 597, 117703. [Google Scholar] [CrossRef]
- Adams, J.S.; Itta, A.K.; Zhang, C.; Wenz, G.B.; Sanyal, O.; Koros, W.J. New insights into structural evolution in carbon molecular sieve membranes during pyrolysis. Carbon 2019, 141, 238–246. [Google Scholar] [CrossRef]
- Sazali, N.; Salleh, W.N.W.; Ismail, A.F.; Kadirgama, K.; Othman, F.E.C.; Ismail, N.H. Impact of stabilization environment and heating rates on P84 co-polyimide/nanocrystaline cellulose carbon membrane for hydrogen enrichment. Int. J. Hydrog. Energy 2019, 44, 20924–20932. [Google Scholar] [CrossRef]
- Wang, Z.; Ren, H.; Zhang, S.; Zhang, F.; Jin, J. Carbon Molecular Sieve Membranes Derived from Troger’s Base-Based Microporous Polyimide for Gas Separation. ChemSusChem 2018, 11, 916–923. [Google Scholar] [CrossRef]
- Kiyono, M.; Williams, P.J.; Koros, W.J. Effect of pyrolysis atmosphere on separation performance of carbon molecular sieve membranes. J. Membr. Sci. 2010, 359, 2–10. [Google Scholar] [CrossRef]
- Kiyono, M.; Williams, P.J.; Koros, W.J. Effect of polymer precursors on carbon molecular sieve structure and separation performance properties. Carbon 2010, 48, 4432–4441. [Google Scholar] [CrossRef]
- Liu, J.; Xiao, Y.; Chung, T.-S. Flexible thermally treated 3D PIM-CD molecular sieve membranes exceeding the upper bound line for propylene/propane separation. J. Mater. Chem. A 2017, 5, 4583–4595. [Google Scholar] [CrossRef] [Green Version]
- Hatori, H.; Kobayashi, T.; Hanzawa, Y.; Yamada, Y.; Iimura, Y.; Kimura, T.; Shiraishi, M. Mesoporous Carbon Membranes from Polyimide Blended with Poly(ethylene glycol). J. Appl. Polym. Sci. 2001, 79, 836–841. [Google Scholar] [CrossRef]
- Ismail, A.F.; David, L.I.B. A review on the latest development of carbon membranes for gas separation. J. Membr. Sci. 2001, 193, 1–18. [Google Scholar] [CrossRef]
- Ozaki, J.; Endo, N.; Ohizumi, W.; Igarashi, K.; Nakahara, M.; Oya, A.; Yoshida, S.; Iizuka, T. Novel preparation method for the production of mesoporous carbon fiber from a polymer blend. Carbon 1997, 35, 1031–1033. [Google Scholar] [CrossRef]
- Kim, Y.K.; Park, H.B.; Lee, Y.M. Carbon molecular sieve membranes derived from thermally labile polymer containing blend polymers and their gas separation properties. J. Membr. Sci. 2004, 243, 9–17. [Google Scholar] [CrossRef]
- Itta, A.K.; Tseng, H.-H.; Wey, M.-Y. Fabrication and characterization of PPO/PVP blend carbon molecular sieve membranes for H2/N2 and H2/CH4 separation. J. Membr. Sci. 2011, 372, 387–395. [Google Scholar] [CrossRef]
- Lee, H.-J.; Suda, H.; Haraya, K. Preparation of Carbon Membranes Derived from Polymer Blends in the Presence of a Thermally Labile Polymer. Sep. Sci. Technol. 2007, 42, 59–71. [Google Scholar] [CrossRef]
- Lo, C.-T.; Seifert, S.; Thiyagarajan, P.; Narasimhan, B. Effect of Polydispersity on the Phase Behavior of Polymer Blends. Macromol. Rapid Commun. 2005, 26, 533–536. [Google Scholar] [CrossRef]
- Vanhee, S.; Koningsveld, R.; Berghmans, H.; Šolc, K.; Stockmayer, W.H. Thermodynamic Stability of Immiscible Polymer Blends. Macromolecules 2000, 33, 3924–3931. [Google Scholar] [CrossRef]
- Gerard, H.; Higgins, J.S.; Clarke, N. Shear-Induced Demixing in Polystyrene/Poly(vinyl methyl ether) Blends. 1. Early Stages of Shear-Induced Demixing. Macromolecules 1999, 32, 5411–5422. [Google Scholar] [CrossRef]
- Lee, H.-J.; Suda, H.; Haraya, K.; Moon, S.-H. Gas permeation properties of carbon molecular sieving membranes derived from the polymer blend of polyphenylene oxide (PPO)/polyvinylpyrrolidone (PVP). J. Membr. Sci. 2007, 296, 139–146. [Google Scholar] [CrossRef]
- Kim, Y.K.; Park, H.B.; Lee, Y.M. Gas separation properties of carbon molecular sieve membranes derived from polyimide/polyvinylpyrrolidone blends: Effect of the molecular weight of polyvinylpyrrolidone. J. Membr. Sci. 2005, 251, 159–167. [Google Scholar] [CrossRef]
- Giel, V.; Moravkova, Z.; Peter, J.; Trchova, M. Thermally treated polyaniline/polybenzimidazole blend membranes: Structural changes and gas transport properties. J. Membr. Sci. 2017, 537, 315–322. [Google Scholar] [CrossRef]
- Fu, Y.-J.; Hu, C.-C.; Lin, D.-W.; Tsai, H.-A.; Huang, S.-H.; Hung, W.-S.; Lee, K.-R.; Lai, J.-Y. Adjustable microstructure carbon molecular sieve membranes derived from thermally stable polyetherimide/polyimide blends for gas separation. Carbon 2017, 113, 10–17. [Google Scholar] [CrossRef]
- Zhang, X.; Hu, H.; Zhu, Y.; Zhu, S. Carbon molecular sieve membranes derived from phenol formaldehyde novolac resin blended with poly(ethylene glycol). J. Membr. Sci. 2007, 289, 86–91. [Google Scholar] [CrossRef]
- Giel, V.; Kredatusová, J.; Trchová, M.; Brus, J.; Žitka, J.; Peter, J. Polyaniline/polybenzimidazole blends: Characterisation of its physico-chemical properties and gas separation behaviour. Eur. Polym. J. 2016, 77, 98–113. [Google Scholar] [CrossRef]
- Gallagher, P.W. Corn ethanol growth in the USA without adverse foreign land-use change: Defining limits and devising policies. Biofuels, Bioprod. Biorefining 2010, 4, 296–309. [Google Scholar] [CrossRef]
- Helberg, R.M.L.; Dai, Z.; Ansaloni, L.; Deng, L. PVA/PVP blend polymer matrix for hosting carriers in facilitated transport membranes: Synergistic enhancement of CO2 separation performance. Green Energy Environ. 2020, 5, 59–68. [Google Scholar] [CrossRef]
- Sazali, N.; Salleh, W.N.W.; Ismail, A.F.; Nordin, N.A.H.M.; Ismail, N.H.; Mohamed, M.A.; Aziz, F.; Yusof, N.; Jaafar, J. Incorporation of thermally labile additives in carbon membrane development for superior gas permeation performance. J. Nat. Gas Sci. Eng. 2018, 49, 376–384. [Google Scholar] [CrossRef]
- Escorial, L.; de la Viuda, M.; Rodríguez, S.; Tena, A.; Marcos, A.; Palacio, L.; Prádanos, P.; Lozano, A.E.; Hernández, A. Partially pyrolized gas-separation membranes made from blends of copolyetherimides and polyimides. Eur. Polym. J. 2018, 103, 390–399. [Google Scholar] [CrossRef]
- Jusoh, N.; Yeong, Y.F.; Lau, K.K.; Shariff, A.M. Fabrication of silanated zeolite T/6FDA-durene composite membranes for CO2/CH4 separation. J. Clean. Prod. 2017, 166, 1043–1058. [Google Scholar] [CrossRef]
- Suo, X.; Cao, Z.; Yu, Y.; Liu, Y. Dynamic self-stiffening in polyacrylonitrile/thermoplastic polyurethane composites. Compos. Sci. Technol. 2020, 198, 108256. [Google Scholar] [CrossRef]
- Hong, S.H.; Cho, Y.; Kang, S.W. Highly porous and thermally stable cellulose acetate to utilize hydrated glycerin. J. Ind. Eng. Chem. 2020, 91, 79–84. [Google Scholar] [CrossRef]
- Ali, S.; Khatri, Z.; Oh, K.W.; Kim, I.-S.; Kim, S.H. Zein/cellulose acetate hybrid nanofibers: Electrospinning and characterization. Macromol. Res. 2014, 22, 971–977. [Google Scholar] [CrossRef]
- Qiu, W.; Zhang, K.; Li, F.S.; Zhang, K.; Koros, W.J. Gas separation performance of carbon molecular sieve membranes based on 6FDA-mPDA/DABA (3:2) polyimide. ChemSusChem 2014, 7, 1186–1194. [Google Scholar] [CrossRef]
- Akbarzadeh, E.; Shockravi, A.; Vatanpour, V. High performance compatible thiazole-based polymeric blend cellulose acetate membrane as selective CO2 absorbent and molecular sieve. Carbohydr. Polym. 2021, 252, 117215. [Google Scholar] [CrossRef]
- Santos-Sauceda, I.; Castillo-Ortega, M.M.; del Castillo-Castro, T.; Armenta-Villegas, L.; Ramírez-Bon, R. Electrospun cellulose acetate fibers for the photodecolorization of methylene blue solutions under natural sunlight. Polym. Bull. 2020, 78, 4419–4438. [Google Scholar] [CrossRef]
- Zhang, B.; Yang, C.; Liu, S.; Wu, Y.; Wang, T.; Qiu, J. The positive/negative effects of bentonite on O2/N2 permeation of carbon molecular sieving membranes. Microporous Mesoporous Mater. 2019, 285, 142–149. [Google Scholar] [CrossRef]
- Jiao, W.; Ban, Y.; Shi, Z.; Jiang, X.; Li, Y.; Yang, W. Gas separation performance of supported carbon molecular sieve membranes based on soluble polybenzimidazole. J. Membr. Sci. 2017, 533, 1–10. [Google Scholar] [CrossRef]
- Omidvar, M.; Nguyen, H.; Huang, L.; Doherty, C.; Hill, A.J.; Stafford, C.M.; Feng, X.; Swihart, M.T.; Lin, H. Unexpectedly Strong Size-Sieving Ability in Carbonized Polybenzimidazole for Membrane H2/CO2 Separation. ACS Appl. Mater. Interfaces 2019, 11, 47365–47372. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Koros, W.J. Ultraselective Carbon Molecular Sieve Membranes with Tailored Synergistic Sorption Selective Properties. Adv. Mater. 2017, 29, 29. [Google Scholar] [CrossRef] [PubMed]
- Rungta, M.; Wenz, G.B.; Zhang, C.; Xu, L.; Qiu, W.; Adams, J.S.; Koros, W.J. Carbon molecular sieve structure development and membrane performance relationships. Carbon 2017, 115, 237–248. [Google Scholar] [CrossRef] [Green Version]













| Composite Membranes | 350 °C Treatment | 600 °C Treatment | Mass Fraction (wt.%) | |
|---|---|---|---|---|
| PI | CA | |||
| PI | PI-350 | PI-600 | 100 | 0 |
| CA0.5 | CA0.5-350 | CA0.5-600 | 99.5 | 0.5 |
| CA1 | CA1-350 | CA1-600 | 99 | 1 |
| CA5 | CA5-350 | CA5-600 | 95 | 5 |
| CA10 | CA10-350 | CA10-600 | 90 | 10 |
| CA20 | CA20-350 | CA20-600 | 80 | 20 |
| CA | CA-350 | CA-600 | 0 | 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Xu, S.; Zhao, B.; Yu, Y.; Liu, Y. The Phase Structural Evolution and Gas Separation Performances of Cellulose Acetate/Polyimide Composite Membrane from Polymer to Carbon Stage. Membranes 2021, 11, 618. https://doi.org/10.3390/membranes11080618
Li H, Xu S, Zhao B, Yu Y, Liu Y. The Phase Structural Evolution and Gas Separation Performances of Cellulose Acetate/Polyimide Composite Membrane from Polymer to Carbon Stage. Membranes. 2021; 11(8):618. https://doi.org/10.3390/membranes11080618
Chicago/Turabian StyleLi, Haojie, Shan Xu, Bingyu Zhao, Yuxiu Yu, and Yaodong Liu. 2021. "The Phase Structural Evolution and Gas Separation Performances of Cellulose Acetate/Polyimide Composite Membrane from Polymer to Carbon Stage" Membranes 11, no. 8: 618. https://doi.org/10.3390/membranes11080618