Evaluation of Electrodialysis Desalination Performance of Novel Bioinspired and Conventional Ion Exchange Membranes with Sodium Chloride Feed Solutions
Abstract
:1. Introduction
1.1. Background
1.2. Objectives
2. Materials and Methods
2.1. Experimental Plan and Variables
2.2. Experimental System and Chemicals
2.3. Electrodialysis (ED) Stack
2.4. Ion Exchange Membranes (IEMs)
- Neosepta AMX/CMX,
- PCA PCSA/PCSK,
- Fujifilm Type 1 AEM/CEM,
- SUEZ AR204SZRA/ CR67HMR,
- Ralex AMH-PES/ CMH‑PES,
- Neosepta AMX/Bare Polycarbonate membrane (Polycarb), and
- Neosepta AMX/Sandia novel bioinspired cation exchange membrane (SandiaCEM).
2.5. Experimental Procedure
- Experimental and pre-rinse solutions (same as experimental concentration) were prepared for electrodialysis system equilibration.
- Pre-rinse solutions from the three process streams were circulated, and the experimental DC voltage was applied at the electrodes to approach the equilibration of the membranes with the solution.
- After evacuating the pre-rinse solution, the electrodialysis apparatus was loaded with the experimental solutions.
- The experiment was performed with full data acquisition.
- Acquired data were analyzed to determine ED desalination performance.
2.6. Data Acquisition and Control Hardware
2.7. Data Analysis
2.8. Calculation Methods
2.8.1. Electrode Voltage Loss
2.8.2. Power and Specific Energy Consumption
2.8.3. Current Density
2.8.4. Limiting Current Density and Limiting Polarization Parameter
2.8.5. Current Efficiency
2.8.6. Salinity Reduction
2.8.7. Water Transport
3. Results
3.1. Evaluation of Limiting Current Density and Areal Resistance
3.2. Evaluation of Current Density and Current Efficiency
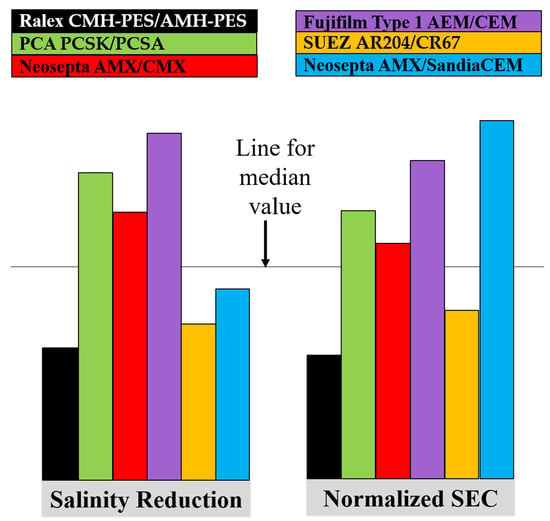
3.3. Evaluation of Salinity Reduction and Normalized Specific Energy Consumption
3.4. Evaluation of Water Flux by Osmosis
4. Conclusions and Recommendations
- The limiting current density (LCD) of an ED stack with Neosepta AMX/CMX membranes, feed solution of 1 to 10 g/L, and superficial velocity of 2 to 8 cm/s ranged from 50 to 600 A/m2, increasing with salinity and increasing with superficial velocity. The voltage application required to achieve LCD ranged from 0.9 to 1.4 Volts per cell pair, and the corresponding areal resistance per cell pair at LCD ranged from 22 to 183 Ω cm2. The limiting polarization parameter ranged from 0.66 to 5.3 A/m2 per meq/L.
- Average current efficiency was observed to decrease with increasing feed salinity for all the membranes. The average current efficiency for a given membrane and salinity combination increased slightly with increasing stack voltage and increasing velocity. Generally, for a given feed concentration and voltage application the current efficiency decreased in the following order (i.e., from greatest efficiency to least efficiency): Fujifilm Type 1 AEM/CEM, PCA PCSK/PCSA, Neosepta AMX/CMX, SUEZ AR204/CR67, Ralex CMH-PES/AMH-PES, AMX/SandiaCEM, and AMX/Polycarb.
- The fractional salinity reduction was observed to decrease with increasing feed salinity for all the membranes, but for a given membrane and feed salinity, the salinity reduction increased significantly with increasing stack voltage and increasing velocity. Generally, the Ralex CMH‑PES/AMH-PES, AMX/SandiaCEM, and AMX/Polycarb membranes were on the lower end of salinity reduction, and Fujifilm Type 1 AEM/CEM showed the greatest salinity reduction for a given feed concentration (3 and 35 g/L) and voltage application. The rest of the membranes showed quite similar performance in salinity reduction, with slightly more differentiation at lower feed concentrations.
- The normalized specific energy consumption (nSEC, kWh/m3 per eq/L removed) was observed to increase with increasing feed salinity for all the membranes. The nSEC for a given membrane and salinity combination increased significantly with increasing stack voltage and increased slightly with increasing velocity. Generally, the Ralex CMH-PES/AMH-PES membranes consumed the least energy but AMX/SandiaCEM and AMX/Polycarb membranes were on the higher end of energy consumption compared to the other membranes for a given feed concentration (3 and 35 g/L) and voltage application. The rest of the membranes showed quite similar performance from a nSEC perspective, with slightly more differentiation at higher feed concentration.
- Water flux by osmosis was observed to increase with the increase of concentration difference (i.e., osmotic pressure difference for a given IEM and superficial velocity). Generally, the osmotic water flux increased in the following order (i.e., from least osmotic flux to greatest osmotic flux): Ralex CMH-PES/AMH-PES (black), Fujifilm Type 1 AEM/CEM (purple), PCA PCSK/PCSA (green), Neosepta AMX/CMX (red), AMX/SandiaCEM (Blue), and SUEZ AR204/CR67.
- The ED desalination performance of the Sandia novel bioinspired cation exchange membrane (SandiaCEM) was observed to be competitive with the commercial cation exchange membranes.
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Banasiak, L.J.; Kruttschnitt, T.W.; Schäfer, A. Desalination using electrodialysis as a function of voltage and salt concentration. Desalination 2007, 205, 38–46. [Google Scholar] [CrossRef] [Green Version]
- Ortiz, J.M.; Expósito, E.; Gallud, F.; García-García, V.; Montiel, V.; Aldaz, A. Desalination of underground brackish waters using an electrodialysis system powered directly by photovoltaic energy. Solar Energy Mater. Sol. Cells 2008, 92, 1677–1688. [Google Scholar] [CrossRef]
- Galama, A.H.; Saakes, M.; Bruning, H.; Rijnaarts, H.H.M.; Post, J.W. Seawater predesalination with electrodialysis. Desalination 2014, 342, 61–69. [Google Scholar] [CrossRef]
- Xu, P.; Capito, M.; Cath, T.Y. Selective removal of arsenic and monovalent ions from brackish water reverse osmosis concentrate. J. Hazard. Mater. 2013, 260, 885–891. [Google Scholar] [CrossRef]
- Dahm, K.; Chapman, M. Produced Water Treatment Primer: Case Studies of Treatment Applications; [US Department of the Interior], Bureau of Reclamation, Technical Service Center: Washington, DC, USA, 2014. [Google Scholar]
- Demircioǧlu, M.; Kabay, N.A.L.A.N.; Ersöz, E.; Kurucaovali, I.; Şafak, Ç.; Gizli, N.İ.L.A.Y. Cost comparison and efficiency modeling in the electrodialysis of brine. Desalination 2001, 136, 317–323. [Google Scholar] [CrossRef]
- El Midaoui, A.; Elhannouni, F.; Taky, M.; Chay, L.; Sahli, M.A.M.; Echihabi, L.; Hafsi, M. Optimization of nitrate removal operation from ground water by electrodialysis. Sep. Apurification Technol. 2002, 29, 235–244. [Google Scholar] [CrossRef]
- Campione, A.; Gurreri, L.; Ciofalo, M.; Micale, G.; Tamburini, A.; Cipollina, A. Electrodialysis for water desalination: A critical assessment of recent developments on process fundamentals, models and applications. Desalination 2018, 434, 121–160. [Google Scholar] [CrossRef]
- Van der Bruggen, B.; Koninckx, A.; Vandecasteele, C. Separation of monovalent and divalent ions from aqueous solution by electrodialysis and nanofiltration. Water Res. 2004, 38, 1347–1353. [Google Scholar] [CrossRef]
- Walker, W.S.; Kim, Y.; Lawler, D.F. Treatment of model inland brackish groundwater reverse osmosis concentrate with electrodialysis—Part I: Sensitivity to superficial velocity. Desalination 2014, 344, 152–162. [Google Scholar] [CrossRef]
- Walker, W.S.; Kim, Y.; Lawler, D.F. Treatment of model inland brackish groundwater reverse osmosis concentrate with electrodialysis—Part II: Sensitivity to voltage application and membranes. Desalination 2014, 345, 128–135. [Google Scholar] [CrossRef]
- Walker, W.S.; Kim, Y.; Lawler, D.F. Treatment of model inland brackish groundwater reverse osmosis concentrate with electrodialysis—Part III: Sensitivity to composition and hydraulic recovery. Desalination 2014, 347, 158–164. [Google Scholar] [CrossRef]
- Percival, S.J.; Small, L.J.; Spoerke, E.D.; Rempe, S.B. Polyelectrolyte layer-by-layer deposition on nanoporous supports for ion selective membranes. RSC Adv. 2018, 8, 32992–32999. [Google Scholar] [CrossRef] [Green Version]
- Percival, S.J.; Russo, S.; Priest, C.; Hill, R.C.; Ohlhausen, J.A.; Small, L.J.; Rempe, S.B.; Spoerke, E.D. Bio-Inspired Incorporation of Phenylalanine Enhances Ionic Selectivity in Layer-by-Layer Deposited Polyelectrolyte Films. J. Phys. Chem. C 2020. Submitted. [Google Scholar]
- Xu, T. Ion exchange membranes: State of their development and perspective. J. Membr. Sci. 2005, 263, 1–29. [Google Scholar] [CrossRef]
- Kemperman, A.J. Handbook Bipolar Membrane Technology; Twente University Press (TUP): Enschede, The Netherlands, 2000. [Google Scholar]
- Available online: http://www.astom-corp.jp/en/index.html (accessed on 4 January 2020).
- Available online: http://www.ameridia.com/html/mbt.html (accessed on 10 January 2020).
- Available online: http://www.pca-gmbh.co, (accessed on 23 January 2020).
- Available online: http://www.ionics.com (accessed on 6 February 2020).
- Available online: http://www.fujifilmmembranes.com (accessed on 6 February 2020).
- Available online: https://www.sterlitech.com (accessed on 8 February 2020).
- Goh, P.S.; Ismail, A.F.; Matsuura, T. Perspective and roadmap of energy-efficient desalination integrated with nanomaterials. Sep. Purif. Rev. 2018, 47, 124–141. [Google Scholar] [CrossRef]
- Firdaous, L.; Malériat, J.P.; Schlumpf, J.P.; Quéméneur, F. Transfer of monovalent and divalent cations in salt solutions by electrodialysis. Sep. Sci. Technol. 2007, 42, 931–948. [Google Scholar] [CrossRef]
- Strathmann, H. Electrodialysis, a mature technology with a multitude of new applications. Desalination 2010, 264, 268–288. [Google Scholar] [CrossRef]
- Strathmann, H. Ion-Exchange Membrane Separation Processes, Membrane Science and Technology Series; Elsevier: Boston, MA, USA, 2004. [Google Scholar]
- He, Y.; Hoi, H.; Abraham, S.; Montemagno, C.D. Highly permeable bioinspired reverse osmosis membrane with amphiphilic peptide stabilized aquaporin as water filtering agent. J. Appl. Polym. Sci. 2018, 135, 46169. [Google Scholar] [CrossRef]
- Kumar, M.; Grzelakowski, M.; Zilles, J.; Clark, M.; Meier, W. Highly permeable polymeric membranes based on the incorporation of the functional water channel protein Aquaporin Z. Proc. Natl. Acad. Sci. USA 2007, 104, 20719–20724. [Google Scholar] [CrossRef] [Green Version]
- Agre, P.; Bonhivers, M.; Borgnia, M.J. The aquaporins, blueprints for cellular plumbing systems. J. Biol. Chem. 1998, 273, 14659–14662. [Google Scholar] [CrossRef] [Green Version]
- Agre, P. Aquaporin water channels (Nobel Lecture). Angew. Chem. Int. Ed. 2004, 43, 4278–4290. [Google Scholar] [CrossRef]
- Borgnia, M.J.; Kozono, D.; Calamita, G.; Maloney, P.C.; Agre, P. Functional reconstitution and characterization of AqpZ, the E. coli water channel protein. J. Mol. Biol. 1999, 291, 1169–1179. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.; Chung, T.S.; Jeyaseelan, K.; Armugam, A. A layer-by-layer self-assembly approach to developing an aquaporin-embedded mixed matrix membrane. RSC Adv. 2013, 3, 473–481. [Google Scholar] [CrossRef]
- ASTOM Corporation, Product Catalogue, Ion exchange membranes, Electrodialyzers, Diffusion Dialyzers. 2017. Available online: http://www.astom-corp.jp/en/catalog/pdf/Astom_Products_Catalogue.pdf (accessed on 7 April 2020).
- Nayar, K.G.; Sundararaman, P.; O’Connor, C.L.; Schacherl, J.D.; Heath, M.L.; Gabriel, M.O.; Winter, A.G. Feasibility study of an electrodialysis system for in-home water desalination in urban India. Dev. Eng. 2017, 2, 38–46. [Google Scholar] [CrossRef]
- Sadrzadeh, M.; Mohammadi, T. Sea water desalination using electrodialysis. Desalination 2008, 221, 440–447. [Google Scholar] [CrossRef]
- Sadrzadeh, M.; Mohammadi, T. Treatment of sea water using electrodialysis: Current efficiency evaluation. Desalination 2009, 249, 279–285. [Google Scholar] [CrossRef]
- Luo, F.; Wang, Y.; Jiang, C.; Wu, B.; Feng, H.; Xu, T. A power free electrodialysis (PFED) for desalination. Desalination 2017, 404, 138–146. [Google Scholar] [CrossRef]
- Krol, J.J.; Wessling, M.; Strathmann, H. Concentration polarization with monopolar ion exchange membranes: Current–voltage curves and water dissociation. J. Membr. Sci. 1999, 162, 145–154. [Google Scholar] [CrossRef]
- Káňavová, N.; Machuča, L.; Tvrzník, D. Determination of limiting current density for different electrodialysis modules. Chem. Pap. 2014, 68, 324–329. [Google Scholar] [CrossRef]
- Lee, H.J.; Strathmann, H.; Moon, S.H. Determination of the limiting current density in electrodialysis desalination as an empirical function of linear velocity. Desalination 2006, 190, 43–50. [Google Scholar] [CrossRef]
- Cappelle, M.; Walker, W.S.; Davis, T.A. Improving desalination recovery using zero discharge desalination (ZDD): A process model for evaluating technical feasibility. Ind. Eng. Chem. Res. 2017, 56, 10448–10460. [Google Scholar] [CrossRef]
- Murray, P. Electrodialysis and Electrodialysis Reversal-Manual of Water Supply Practices, M38; American Water Works Association: Colorado: Denver, Co, USA, 1995. [Google Scholar]
- Vanysek, P. CRC Handbook of Chemistry and Physics, 93rd ed.; CRC Press: Boca Raton, FL, USA, 2012; pp. 5–74. [Google Scholar]
- Landolt-Börnstein: Numerical Values and Functions, 6th ed.; Properties of Matter in their States of Aggregation. Part 7, Electrical Properties II (Electrochemical Systems); Springer: Berlin/Heidelberg, Germany, 1960; Volume II, 959p.
- Kingsbury, R.S.; Zhu, S.; Flotron, S.; Coronell, O. Microstructure determines water and salt permeation in commercial ion-exchange membranes. ACS Appl. Mater. Interfaces 2018, 10, 39745–39756. [Google Scholar] [CrossRef] [PubMed]








| Variables | Discrete Values/Combinations |
|---|---|
| NaCl feed water concentration | 1, 3, 10, 35, 100 g/L |
| Superficial velocity of diluate stream | 2, 4, 8 cm/s (corresponding flow: 15, 30, 60 mL/min) |
| Stack voltage | 0.4, 0.8, 1.2 V/cell-pair |
| Combination of membranes during stack assembly | i. Neosepta AMX & CMX ii. PCA PCSA & PCSK iii. Fujifilm Type 1 AEM & CEM iv. SUEZ AR204SZRA & CR67HMR v. Ralex AMH-PES & CMH-PES vi. Neosepta AMX & bare polycarbonate (Polycarb) vii. Neosepta AMX & Sandia novel bioinspired CEM (SandiaCEM) |
| Membrane | Type | Thickness (mm) | IEC (meq/g) | Areal Resistance (Ω cm2) | Remarks | Ref. |
|---|---|---|---|---|---|---|
| Polycarbonate | - | 0.006 | - | 10.3 | Filtering air/water | [22] |
| Ralex AMH-PES * | AEM | 0.55 Dry | 1.8 | <8 | ED, EDI | [15,20] |
| Ralex CMH-PES * | CEM | 0.45 Dry | 2.2 | <9 | ED, EDI | [15,20] |
| PCA PCSA | AEM | 0.232 | 1.69 | - | Standard ED | [19] |
| PCA PCSK | CEM | 0.098 | 1.25 | - | Standard ED | [19] |
| Neosepta AMX | AEM | 0.12–0.18 | 1.4–1.7 | 2.0–3.5 | High strength | [15,17] |
| Neosepta CMX | CEM | 0.14–0.20 | 1.5–1.8 | 2.0–3.5 | High strength | [15,17] |
| Fujifilm Type 1 AEM | AEM | 0.125 | 1.50 | 1.3 | Water softening | [21] |
| Fujifilm Type 1 CEM | CEM | 0.135 | 1.43 | 2.7 | Water softening | [21] |
| SUEZ AR204SZRA | AEM | 0.48–0.66 | 2.3–2.7 | 6.2–9.3 | EDR | [15,20] |
| SUEZ CR67HMR | CEM | 0.53–0.65 | 2.1–2.45 | 7.0–11.0 | ED | [15,20] |
| Sandia CEM * | CEM | 0.0072 | - | 18.5 | ED, EDR | [13] |
| Membrane | Ralex CMH-PES/AMH-PES | PCA PCSK/PCSA | Neosepta AMX/CMX | Fujifilm Type 1 AEM/CEM | SUEZ AR204/CR67 | AMX/SandiaCEM |
|---|---|---|---|---|---|---|
| Current Density * | min | >med | >med | max | <med | >med |
| Current Efficiency * | <med | >med | >med | max | <med | min |
| Salinity Reduction | min | >med | >med | max | <med | <med |
| Normalized SEC | min | >med | >med | >med | <med | max |
| Water Permeance | min | <med | >med | <med | max | >med |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hyder, A.G.; Morales, B.A.; Cappelle, M.A.; Percival, S.J.; Small, L.J.; Spoerke, E.D.; Rempe, S.B.; Walker, W.S. Evaluation of Electrodialysis Desalination Performance of Novel Bioinspired and Conventional Ion Exchange Membranes with Sodium Chloride Feed Solutions. Membranes 2021, 11, 217. https://doi.org/10.3390/membranes11030217
Hyder AG, Morales BA, Cappelle MA, Percival SJ, Small LJ, Spoerke ED, Rempe SB, Walker WS. Evaluation of Electrodialysis Desalination Performance of Novel Bioinspired and Conventional Ion Exchange Membranes with Sodium Chloride Feed Solutions. Membranes. 2021; 11(3):217. https://doi.org/10.3390/membranes11030217
Chicago/Turabian StyleHyder, AHM Golam, Brian A. Morales, Malynda A. Cappelle, Stephen J. Percival, Leo J. Small, Erik D. Spoerke, Susan B. Rempe, and W. Shane Walker. 2021. "Evaluation of Electrodialysis Desalination Performance of Novel Bioinspired and Conventional Ion Exchange Membranes with Sodium Chloride Feed Solutions" Membranes 11, no. 3: 217. https://doi.org/10.3390/membranes11030217