Integrated Membrane Process for the Treatment and Reuse of Residual Table Olive Fermentation Brine and Anaerobically Digested Sludge Centrate
Abstract
:1. Introduction
2. Materials and Methods
2.1. Wastewater
2.2. Analytical Methods
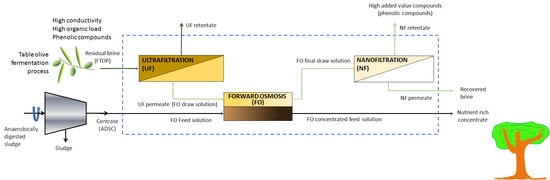
2.3. Integrated Process
2.4. Ultrafiltration Step
2.5. Forward Osmosis Step
2.6. Nanofiltration Step
3. Results and Discussion
3.1. Characterization of Feed Samples
3.2. Ultrafiltration Step
3.3. Forward Osmosis Step
3.4. Nanofiltration Step
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Abou-Elela, S.I.; Kamel, M.M.; Fawzy, M.E. Biological treatment of saline wastewater using a salt-tolerant microorganism. Desalination 2010, 250, 1–5. [Google Scholar] [CrossRef]
- Ferrer-Polonio, E.; Iborra-Clar, A.; Mendoza-Roca, J.A.; Pastor-Alcañiz, L. Fermentation brines from Spanish style green table olives processing: Treatment alternatives before re cycling or recovery operations. J. Chem. Technol. Biotechnol. 2016, 91, 131–137. [Google Scholar] [CrossRef]
- Reid, E.; Liu, X.; Judd, S.J. Effect of high salinity on activated sludge characteristics and membrane permeability in an immersed membrane bioreactor. J. Membr. Sci. 2006, 283, 164–171. [Google Scholar] [CrossRef]
- Ferrer-Polonio, E.; Mendoza-Roca, J.A.; Iborra-Clar, A.; Alonso-Molina, J.L.; Pastor-Alcañiz, L. Comparison of two strategies for the start-up of a biological reactor for the treatment of hypersaline effluents from a table olive packaging industry. Chem. Eng. J. 2015, 273, 595–602. [Google Scholar] [CrossRef]
- Li, H.; Meng, F.P.; Duan, W.Y.; Li, Y.F.; Zheng, Y. Biodegradation of phenol in saline or hypersaline environments by bacteria: A review. Ecotoxicol. Environ. Saf. 2019, 184, 109658. [Google Scholar] [CrossRef]
- Ayed, L.; Asses, N.; Chammem, N.; Ben Othman, N.; Hamdi, M. Advanced oxidation process and biological treatments for table olive processing wastewaters: Constraints and a novel approach to integrated recycling process: A review. Biodegradation 2017, 28, 125–138. [Google Scholar] [CrossRef]
- Ferrer-Polonio, E.; Mendoza-Roca, J.A.; Iborra-Clar, A.; Pastor-Alcañiz, L. Adsorption of raw and treated by membranes fermentation brines from table olives processing for phenolic compounds separation and recovery. J. Chem. Technol. Biotechnol. 2016, 91, 2094–2102. [Google Scholar] [CrossRef]
- El-Abbassi, A.; Kiai, H.; Raiti, J.; Hafidi, A. Application of ultrafiltration for olive processing wastewaters treatment. J. Clean. Prod. 2014, 65, 432–438. [Google Scholar] [CrossRef]
- Carbonell-Alcaina, C.; Álvarez-Blanco, S.; Bes-Piá, M.A.; Mendoza-Roca, J.A.; Pastor-Alcañiz, L. Ultrafiltration of residual fermentation brines from the production of table olives at different operating conditions. J. Clean. Prod. 2018, 189, 662–672. [Google Scholar] [CrossRef]
- Kiai, H.; García-Payo, M.C.; Hafidi, A.; Khayet, M. Application of membrane distillation technology in the treatment of table olive wastewaters for phenolic compounds concentration and high quality water production. Chem. Eng. Process. 2014, 86, 153–161. [Google Scholar] [CrossRef]
- Vu, M.T.; Ansari, A.J.; Hai, F.I.; Nghiem, L.D. Performance of a seawater-driven forward osmosis process for pre-concentrating digested sludge centrate: Organic enrichment and membrane fouling. Environ. Sci. Water Res. Technol. 2018, 4, 1047–1056. [Google Scholar] [CrossRef]
- Soler-Cabezas, J.L.; Mendoza-Roca, J.A.; Vincent-Vela, M.C.; Luján-Facundo, M.J.; Pastor-Alcañiz, L. Simultaneous concentration of nutrients from anaerobically digested sludge centrate and pre-treatment of industrial effluents by forward osmosis. Sep. Purif. Technol. 2018, 193, 289–296. [Google Scholar] [CrossRef]
- Duan, J.; Litwiller, E.; Choi, S.H.; Pinnau, I. Evaluation of sodium lignin sulfonate as draw solute in forward osmosis for desert restoration. J. Membr. Sci. 2014, 453, 463–470. [Google Scholar] [CrossRef]
- Hu, B.; Jiang, M.; Zhao, S.; Ji, X.; Shu, Q.; Tian, B.; He, T.; Zhang, L. Biogas slurry as draw solution of forward osmosis process to extract clean water from micro-polluted water for hydroponic cultivation. J. Membr. Sci. 2019, 576, 88–95. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- De Castro, A.; Brenes, M. Fermentation of washing waters of spanish-style green olive processing. Process Biochem. 2001, 36, 797–802. [Google Scholar] [CrossRef]
- Cheryan, M. Ultrafiltration and Microfiltration Handbook, 2nd ed.; CRC Press: Boca Ratón, FL, USA, 1998. [Google Scholar]
- Luján-Facundo, M.J.; Mendoza-Roca, J.A.; Soler-Cabezas, J.L.; Bes-Piá, A.; Vincent-Vela, M.C.; Pastor-Alcañiz, L. Use of the osmotic membrane bioreactor for the management of tannery wastewater using absorption liquid waste as draw solution. Process Saf. Environ. Protect. 2019, 131, 292–299. [Google Scholar] [CrossRef]
- Ferrer-Polonio, E.; Carbonell-Alcaina, C.; Mendoza-Roca, J.A.; Iborra-Clar, A.; Álvarez-Blanco, S.; Bes-Piá, A.; Pastor-Alcañiz, L. Brine recovery from hypersaline wastewaters from table olive processing by combination of biological treatment and membrane technologies. J. Clean. Prod. 2017, 142, 1377–1386. [Google Scholar] [CrossRef]
- Santafé-Moros, A.; Gozálvez-Zafrilla, J.M. Design of a flat membrane module for fouling and permselectivity studies. In Proceedings of the COMSOL Conference, Paris, France, 29–30 October 2010; pp. 1–7. [Google Scholar]
- Papadaki, E.; Mantzouridou, F.T. Current status and future challenges of table olive processing wastewater valorization. Biochem. Eng. J. 2016, 112, 103–113. [Google Scholar] [CrossRef]
- Carbonell-Alcaina, C.; Corbatón-Báguena, M.J.; Alvarez-Blanco, S.; Bes-Piá, M.A.; Mendoza-Roca, J.A.; Pastor-Alcañiz, L. Determination of fouling mechanisms in polymeric ultrafiltration membranes using residual brines from table olive storage wastewaters as feed. J. Food Eng. 2016, 187, 14–23. [Google Scholar] [CrossRef]
- Sayadi, S.; Allouche, N.; Jaoua, M.; Aloui, F. Detrimental effects of high molecular-mass polyphenols on olive mill wastewater biotreatment. Process Biochem. 2000, 35, 725–735. [Google Scholar] [CrossRef]
- Li, Y.; Xu, Z.; Xie, M.; Zhang, B.; Li, G.; Luo, W. Resource recovery from digested manure centrate: Comparison between conventional and aquaporin thin-film composite forward osmosis membranes. J. Membr. Sci. 2020, 593, 117436. [Google Scholar] [CrossRef]
- Bargeman, G.; Vollenbroek, J.M.; Straatsma, J.; Schroën, C.G.P.H.; Boom, R.M. Nanofiltration of multi-component feeds. Interactions between neutral and charged components and their effect on retention. J. Membr. Sci. 2005, 247, 11–20. [Google Scholar] [CrossRef]
- Koyuncu, I.; Topacik, D.; Wiesner, M.R. Factors influencing flux decline during nanofiltration of solutions containing dyes and salts. Water Res. 2004, 2004 38, 432–440. [Google Scholar] [CrossRef]
- Wiesner, M.; Aptel, P. Mass transport and permeate flux and fouling in pressure-driven processes. In Water Treatment Membrane Handbook; Malleviella, J., Ed.; McGraw-Hill: New York, NY, USA, 1996. [Google Scholar]
- Virga, E.; Spruijt, E.; de Vos, W.M.; Biesheuvel, P.M. Wettability of Amphoteric Surfaces: The Effect of pH and Ionic Strength on Surface Ionization and Wetting. Langmuir 2018, 34, 15174–15180. [Google Scholar] [CrossRef] [Green Version]
- Van der Bruggen, B.; Cornelis, G.; Vandecasteele, C.; Devreese, I. Fouling of nanofiltration and ultrafiltration membranes applied for wastewater regeneration in the textile industry. Desalination 2005, 175, 111–119. [Google Scholar] [CrossRef]
- Spiegler, K.S.; Kedem, O. Thermodynamics of hyperfiltration (reverse osmosis): Criteria for efficient membranes. Desalination 1966, 1, 311–326. [Google Scholar] [CrossRef]








| Ultrafiltration (UF) | Nanofiltration (NF) | |||||||
|---|---|---|---|---|---|---|---|---|
| Forward Osmosis (FO) | ||||||||
| UF Feed (FTOP) | UF Retentate | UF Permeate FO Draw Solution | FO Initial Feed (Digester Centrate) | FO Final Feed (Digester Centrate) | FO Final Draw Solution NF Feed | NF Permeate (15 bar) | NF Permeate (9 bar) | |
| pH | 4.4 ± 0.2 | 4.8 ± 0.1 | 4.5 ± 0.1 | 7.8 ± 0.1 | 8.1 ± 0.1 | 8.8 ± 0.2 | 8.9 ± 0.1 | 8.7 ± 0.1 |
| Conductivity (mS cm−1) | 72.3 ± 0.2 | 79.3 ± 0.2 | 71.5 ± 0.9 | 11.9 ± 0.2 | 23.2 ± 0.2 | 25.7 ± 0.2 | 21.0 ± 0.2 | 24.5 ± 0.1 |
| Color | 0.458 ± 0.002 | 0.558 ± 0.002 | 0.252 ± 0.016 | - | - | 0.838 ± 0.053 | 0.001 ± 0.000 | 0.003 ± 0.001 |
| COD 1 (mg O2 L−1) | 9730 ± 57 | 15650 ± 260 | 7756 ± 67 | 613 ± 28 | 1740 ± 103 | 2663 ± 68 | 301 ± 4 | 331 ± 4 |
| Total nitrogen (mg L−1) | 474.0 ± 28.3 | 475.8 ± 30.1 | 421.2 ± 26.5 | 1087.9 ± 45.4 | 1642.4 ± 45.4 | 654.0 ± 31.2 | 274.0 ± 25.6 | 317.0 ± 24.7 |
| Total phosporous (mg L−1) | 37.2 ± 2.1 | 29.6 ± 1.5 | 28.9 ± 1.6 | 9.7 ± 0.5 | 2.7 ± 0.5 | 7.0 ± 0.2 | N.d 3 | N.d |
| Total ph. comp. 2 (mg·Ty eq. L−1) | 1012.6 ± 24.3 | 1407.3 ± 13.4 | 812.9 ± 30.2 | 63.4 ± 1.6 | 154.7 ± 4.9 | 152.0 ± 27.7 | 23.2 ± 2.0 | 25.9 ± 0.5 |
| UF-FO-NF (15 bar) | UF-FO-NF (9 bar) | UF-NF (15 bar) | |
|---|---|---|---|
| Conductivity (%) | 18.3 ± 1.2 | 4.7 ± 1.0 | 25.4 ± 0.3 |
| Color (%) | 99.9 ± 0.1 | 99.6 ± 0.2 | 91.4 ± 1.2 |
| COD (%) | 88.7 ± 0.4 | 87.6 ± 0.4 | 64.7 ± 0.6 |
| Total nitrogen (%) | 58.1 ± 5.1 | 51.5 ± 5.2 | - |
| Total phosporous (%) | 100.0 ± 0.0 | 100.0 ± 0.0 | - |
| Total phenolic compounds (%) | 84.7 ± 3.7 | 83.0 ± 3.8 | 35.2 ± 2.4 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carbonell-Alcaina, C.; Soler-Cabezas, J.L.; Bes-Piá, A.; Vincent-Vela, M.C.; Mendoza-Roca, J.A.; Pastor-Alcañiz, L.; Álvarez-Blanco, S. Integrated Membrane Process for the Treatment and Reuse of Residual Table Olive Fermentation Brine and Anaerobically Digested Sludge Centrate. Membranes 2020, 10, 253. https://doi.org/10.3390/membranes10100253
Carbonell-Alcaina C, Soler-Cabezas JL, Bes-Piá A, Vincent-Vela MC, Mendoza-Roca JA, Pastor-Alcañiz L, Álvarez-Blanco S. Integrated Membrane Process for the Treatment and Reuse of Residual Table Olive Fermentation Brine and Anaerobically Digested Sludge Centrate. Membranes. 2020; 10(10):253. https://doi.org/10.3390/membranes10100253
Chicago/Turabian StyleCarbonell-Alcaina, Carlos, Jose Luis Soler-Cabezas, Amparo Bes-Piá, María Cinta Vincent-Vela, Jose Antonio Mendoza-Roca, Laura Pastor-Alcañiz, and Silvia Álvarez-Blanco. 2020. "Integrated Membrane Process for the Treatment and Reuse of Residual Table Olive Fermentation Brine and Anaerobically Digested Sludge Centrate" Membranes 10, no. 10: 253. https://doi.org/10.3390/membranes10100253