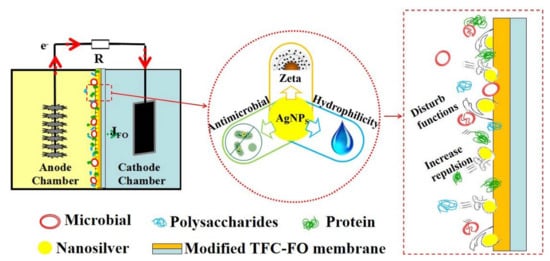
Performance Improvement and Biofouling Mitigation in Osmotic Microbial Fuel Cells via In Situ Formation of Silver Nanoparticles on Forward Osmosis Membrane
Abstract
:1. Introduction
2. Materials and Methods
2.1. In Situ Formation of AgNPs on TFC-FO Membrane
2.2. Analytical Methods
2.3. Set-up and Operating Conditions
3. Results and Discussion
3.1. In Situ AgNP Modified FO Membrane Analyses
3.2. Electricity Generation of OsMFC
3.3. Removal of Contaminants
3.4. Performance of the FO Membrane
3.5. Mechanisms of Fouling Mitigation by AgNPs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, F.; Brastad, K.S.; He, Z. Integrating forward osmosis into microbial fuel cells for wastewater treatment, water extraction and bioelectricity generation. Environ. Sci. Technol. 2011, 45, 6690–6696. [Google Scholar] [CrossRef] [PubMed]
- Li, W.W.; Yu, H.Q.; He, Z. Towards sustainable wastewater treatment by using microbial fuel cells-centered technologies. Energ. Environ. Sci. 2014, 7, 911–924. [Google Scholar] [CrossRef] [Green Version]
- He, Z. One more function for microbial fuel cells in treating wastewater: Producing high-quality water. Chemik 2012, 66, 7–10. [Google Scholar]
- Ge, Z.; He, Z. Effects of draw solutions and membrane conditions on electricity generation and water flux in osmotic microbial fuel cells. Bioresour. Technol. 2012, 109, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Ge, Z.; Ping, Q.Y.; Xiao, L.; He, Z. Reducing effluent discharge and recovering bioenergy in an osmotic microbial fuel cell treating domestic wastewater. Desalination 2013, 312, 52–59. [Google Scholar] [CrossRef]
- Lu, Y.B.; Qin, M.H.; Yuan, H.Y.; Reesh, I.A.; He, Z. When bioelectrochemical systems meet forward osmosis: Accomplishing wastewater treatment and reuse through synergy. Water 2014, 7, 38–50. [Google Scholar] [CrossRef]
- Lu, Y.Q.; Bian, X.; Wang, X.H.; Ren, Y.P.; Li, X.F. Simultaneously recovering electricity and water from wastewater by osmotic microbial fuel cells: Performance and membrane fouling. Front. Environ. Sci. Eng. 2018, 12, 45–54. [Google Scholar] [CrossRef]
- Werner, C.M.; Logan, B.E.; Saikaly, P.E.; Amy, G.L. Wastewater treatment, energy recovery and desalination using a forward osmosis membrane in an aircathode microbial osmotic fuel cell. J. Membr. Sci. 2013, 428, 116–122. [Google Scholar] [CrossRef]
- Pardeshi, P.M.; Mungray, A. High flux layer by layer polyelectrolyte FO membrane: Toward enhanced performance for osmotic microbial fuel cell. Int. J. Polym. Mater. 2014, 63, 595–601. [Google Scholar] [CrossRef]
- Zhu, X.Z.; Zhang, F.; Li, W.W.; Li, J.; Li, L.L.; Yu, H.Q.; Huang, M.S.; Huang, T.Y. Insights into enhanced current generation of an osmotic microbial fuel cell under membrane fouling condition. J. Membr. Sci. 2016, 504, 40–46. [Google Scholar] [CrossRef]
- Xu, J.; Sheng, G.P.; Luo, H.W.; Li, W.W.; Wang, L.F.; Yu, H.Q. Fouling of proton exchange membrane (PEM) deteriorates the performance of microbial fuel cell. Water Res. 2012, 46, 1817–1824. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.J.; Chae, K.J.; Ajayi, F.F.; Kim, K.Y.; Yu, H.W.; Kim, C.W.; Kim, I.S. Effects of biofouling on ion transport through cation exchange membranes and microbial fuel cell performance. Bioresour. Technol. 2011, 102, 298–303. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.Y.; Hurt, R.H. Ion release kinetics and particle persistence in aqueous nanosilver colloids. Environ. Sci. Technol. 2010, 44, 2169–2175. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.L.; Rosenfield, E.; Hu, M.; Mi, B.X. Direct observation of bacterial deposition on and detachment from nanocomposite membranes embedded with silver nanoparticles. Water Res. 2013, 47, 2949–2958. [Google Scholar] [CrossRef]
- Dong, C.X.; Wu, J.H.; Wang, Y.; Wang, J.X.; Wang, S.C. A green strategy to immobilize silver nanoparticles onto reverse osmosis membrane for enhanced anti-biofouling property. Desalination 2017, 401, 32–41. [Google Scholar] [CrossRef]
- Zhao, Y.X.; Wang, X.H.; Wang, Z.W.; Li, X.F.; Ren, Y.P. Nanoparticle fouling and its combination with organic fouling during forward osmosis process for silver nanoparticles removal from simulated wastewater. Sci. Rep. 2016, 6, 25859–25869. [Google Scholar] [CrossRef] [Green Version]
- Yang, E.; Chae, K.J.; Alayande, A.B.; Kim, K.Y.; Kim, I.S. Concurrent performance improvement and biofouling mitigation in osmotic microbial fuel cells using a silver nanoparticle-polydopamine coated forward osmosis membrane. J. Membr. Sci. 2016, 513, 217–225. [Google Scholar] [CrossRef]
- Wu, H.; Liu, Y.; Huang, J.; Mao, L.; Chen, J.; Li, M. Preparation and characterization of antifouling and antibacterial polysulfone ultrafiltration membranes incorporated with a silver-polydopamine nanohybrid. J. Appl. Polym. Sci. 2018, 135, 46430. [Google Scholar] [CrossRef]
- Chew, N.; Zhang, Y.; Goh, K.; Ho, J.S.; Wang, R. Hierarchically structured Janus membrane surfaces for enhanced membrane distillation performance. ACS Appl. Mater. Interfaces. 2019, 11, 25524–25534. [Google Scholar] [CrossRef]
- Huang, L.; Zhao, S.; Wang, Z.; Wu, J.; Wang, S. In situ immobilization of silver nanoparticles for improving permeability, antifouling and anti-bacterial properties of ultrafiltration membrane. J. Membr. Sci. 2016, 499, 269–281. [Google Scholar] [CrossRef]
- Tang, L.; Livi, K.J.T.; Chen, K.L. Polysulfone membranes modified with bioinspired polydopamine and silver nanoparticles formed in situ to mitigate biofouling. Environ. Sci. Technol. Lett. 2015, 2, 59–65. [Google Scholar] [CrossRef]
- Chung, T.S.; Luo, L.; Wan, C.F.; Cui, Y.; Amy, G. What is next for forward osmosis (FO) and pressure retarded osmosis. Sep. Purif. Technol. 2015, 156, 856–860. [Google Scholar] [CrossRef] [Green Version]
- Yip, N.Y.; Tiraferri, A.; Phillip, W.A.; Schiffman, J.D.; Elimelech, M. High performance thin-film composite forward osmosis membrane. Environ. Sci. Technol. 2010, 44, 3812–3818. [Google Scholar] [CrossRef] [PubMed]
- Xiao, T.T.; Nghiem, L.D.; Song, J.F.; Bao, R.Y.; Li, X.M.; He, T. Phenol rejection by cellulose triacetate and thin film composite forward osmosis membranes. Sep. Purif. Technol. 2017, 186, 45–54. [Google Scholar] [CrossRef]
- Wang, X.H.; Zhang, J.F.; Chang, V.W.C.; She, Q.H.; Tang, C.Y.Y. Removal of cytostatic drugs from wastewater by an anaerobic osmotic membrane bioreactor. Chem. Eng. J. 2018, 339, 153–161. [Google Scholar] [CrossRef]
- Liao, Y.; Wang, Y.Q.; Feng, X.X.; Wang, W.C.; Xu, F.J.; Zhang, L.Q. Antibacterial surfaces through dopamine functionalization and silver nanoparticle immobilization. Mater. Chem. Phys. 2010, 121, 534–540. [Google Scholar] [CrossRef]
- Wang, X.H.; Chen, Y.; Yuan, B.; Li, X.F.; Ren, Y.P. Impacts of sludge retention time on sludge characteristics and membrane fouling in a submerged osmotic membrane bioreactor. Bioresour. Technol. 2014, 161, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.H.; Hu, T.Z.; Wang, Z.W.; Li, X.F.; Ren, Y.P. Permeability recovery of fouled forward osmosis membranes by chemical cleaning during a long-term operation of anaerobic osmotic membrane bioreactors treating low-strength wastewater. Water Res. 2017, 123, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wang, X.H.; Xie, M.; Wang, Z.W.; Li, X.F.; Ren, Y.P. In situ extracting organic-bound calcium: A novel approach to mitigating organic fouling in forward osmosis treating wastewater via gradient diffusion thin-films. Water Res. 2019, 156, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wang, X.H.; Xie, M.; Wang, H.L.; Li, X.F.; Ren, Y.P. EDTA-based adsorption layer for mitigating FO membrane fouling via in situ removing calcium binding with organic foulants. J. Membr. Sci. 2019, 578, 95–102. [Google Scholar] [CrossRef]
- Pan, S.F.; Zhu, M.P.; Chen, J.P.; Yuan, Z.H.; Zhong, L.B.; Zheng, L.M. Separation of tetracycline from wastewater using forward osmosis process with thin film composite membrane—Implications for antibiotics recovery. Sep. Purif. Technol. 2015, 153, 76–83. [Google Scholar] [CrossRef]
- Sasson, M.B.; Lu, X.L.; Zeev, E.B.; Zodrow, K.R.; Nejati, S.; Qi, G.G. In situ formation of silver nanoparticles on thin-film composite reverse osmosis membranes for biofouling mitigation. Water Res. 2014, 62, 260–270. [Google Scholar] [CrossRef]
- Wang, X.H.; Zhao, Y.X.; Yuan, B.; Wang, Z.W.; Li, X.F.; Ren, Y.P. Comparison of biofouling mechanisms between cellulose triacetate (CTA) and thin-film composite (TFC) polyamide forward osmosis membranes in osmotic membrane bioreactors. Bioresour. Technol. 2016, 202, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Perreault, F.; Jaramillo, H.; Xie, M.; Ude, M.; Elimelech, M. Biofouling mitigation in forward osmosis using graphene oxide functionalized thin-film composite membranes. Environ. Sci. Technol. 2016, 50, 5840–5848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Jin, X.; Cao, B.; Tang, C.Y. Bactericidal activity of silver nanoparticles in environmentally relevant freshwater matrices: Influences of organic matter and chelating agent. J. Environ. Chem. Eng. 2014, 2, 525–531. [Google Scholar] [CrossRef]
- Liu, Q.; Qiu, G.L.; Zhou, Z.Z.; Li, J.G.; Amy, G.L.; Xie, J.P.; Lee, J.Y. An effective design of electrically conducting thin-film composite (TFC) membranes for bio and organic fouling control in forward osmosis (FO). Environ. Sci. Technol. 2016, 50, 10596–10605. [Google Scholar] [CrossRef]
- Wu, S.M.; Qian, G.R.; He, Z. Examination of inorganic-based draw solutes and mitigation of their reverse solute flux in osmotic microbial fuel cell. J. Chem. Technol. Biot. 2018, 9, 2107–2114. [Google Scholar] [CrossRef]
- N.E.P.A. Chinese. Water and Wastewater Monitoring Methods, 4th ed.; Chinese Environmental Science Publishing House: Beijing, China, 2002. [Google Scholar]
- Liu, J.M.; Wang, X.H.; Wang, Z.W.; Lu, Y.Q.; Li, X.F.; Ren, Y.P. Integrating microbial fuel cells with anaerobic acidification and forward osmosis membrane for enhancing bio-electricity and water recovery from low-strength wastewater. Water Res. 2016, 110, 74–82. [Google Scholar] [CrossRef]
- Zhang, F.; Jacobson, K.S.; Torres, P.; He, Z. Effects of anolyte recirculation rates and catholytes on electricity generation in a liter-scale upflow microbial fuel cell. Energ. Environ. Sci. 2010, 3, 1347–1352. [Google Scholar] [CrossRef]
- Qin, M.; Abureesh, I.M.; He, Z. Effects of current generation and electrolyte pH on reverse salt flux across thin film composite membrane in osmotic microbial fuel cells. Water Res. 2016, 105, 583–590. [Google Scholar] [CrossRef] [Green Version]
- Zodrow, K.; Brunet, L.; Mahendra, S.; Li, D.; Zhang, A.; Li, Q.L.; Alvarez, P.J.J. Polysulfone ultrafiltration membranes impregnated with silver nanoparticles show improved biofouling resistance and virus removal. Water Res. 2009, 43, 715–723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, E.S.; Wang, G.H.; El-Din, M.G.; Liu, Y. Development of nanosilver and multi-walled carbon nanotubes thin-film nanocomposite membrane for enhanced water treatment. J. Membr. Sci. 2012, 394–395, 37–48. [Google Scholar] [CrossRef]
- Veluchamy, P.; Sivakumar, P.M.; Doble, M. Green synthesis of protein stabilized silver nanoparticles using Pseudomonas fluorescens, a marine bacterium, and its biomedical applications when coated on polycaprolactam. Ind. Eng. Chem. Res. 2012, 51, 5230–5239. [Google Scholar] [CrossRef]
- Yin, J.; Yang, Y.; Hu, Z.Q.; Deng, B.L. Attachment of silver nanoparticles (AgNPs) onto thin-film composite (TFC) membranes through covalent bonding to reduce membrane biofouling. J. Membr. Sci. 2013, 441, 73–82. [Google Scholar] [CrossRef]
- Rana, D.; Matsuura, T. Surface modifications for antifouling membranes. Chem. Rev. 2010, 110, 2448–2471. [Google Scholar] [CrossRef]
- Kochkodan, V.; Hilal, N. A comprehensive review on surface modified polymer membranes for biofouling mitigation. Desalination 2015, 356, 187–207. [Google Scholar] [CrossRef]
- Sadowski, Z.; Maliszewska, I.; Grochowalska, B.; Polowczyk, I.; Kozlecki, T. Synthesis of silver nanoparticles using microorganisms. Mater. Sci. Poland 2008, 26, 419–424. [Google Scholar] [CrossRef]
- Periasamy, M.; Thirumalaikumar, P. Methods of enhancement of reactivity and selectivity of sodium borohydride for applications in organic synthesis. J. Org. Chem. 2000, 609, 137–151. [Google Scholar] [CrossRef]
- Qin, M.; Hynes, E.A.; Reesh, I.A.; He, Z. Ammonium removal from synthetic wastewater promoted by current generation and water flux in an osmotic microbial fuel cell. J. Clean. Prod. 2017, 149, 856–862. [Google Scholar] [CrossRef] [Green Version]
- Mi, B.X.; Elimelech, M. Gypsum scaling and cleaning in forward osmosis: Measurements and mechanisms. Environ. Sci. Technol. 2010, 44, 2022–2028. [Google Scholar] [CrossRef]
- Wang, H.L.; Wang, X.H.; Meng, F.G.; Li, X.F.; Ren, Y.P.; She, Q.H. Effect of driving force on the performance of anaerobic osmotic membrane bioreactors: New insight into enhancing water flux of FO membrane via controlling driving force in a two-stage pattern. J. Membr. Sci. 2019, 569, 41–47. [Google Scholar] [CrossRef]
- Li, J.X.; Sanderson, R.D.; Jacobs, E.P. Ultrasonic cleaning of nylon microfiltration membranes fouled by kraft paper mill effluent. J. Membr. Sci. 2002, 205, 247–257. [Google Scholar] [CrossRef]
- Kwan, S.E.; Zeev, E.B.; Elimelech, M. Biofouling in forward osmosis and reverse osmosis: Measurements and mechanism. J. Membr. Sci. 2015, 493, 703–708. [Google Scholar] [CrossRef]








| Characteristics | Pristine Membrane | Modified Membrane |
|---|---|---|
| Contact angle (degree) | 46.6 ± 1.3 | 33.6 ± 1.1 |
| Surface charge (mV) | −19.10 ± 0.21 | −34.56 ± 0.40 |
| Surface roughness (nm) | 38.4 ± 1 | 33.3 ± 2 |
| Water flux (LMH) | 12.5 ± 0.5 | 8.5 ± 0.5 |
| Reverse salt flux (10−8 m/s) | 9.4 ± 0.3 | 2.7± 0.1 |
| JS/JW | 0.75 ± 0.12 | 0.31 ± 0.05 |
| Bacteria colonies (107 CFU/mL) | 2.05 ± 0.07 | 1.55 ± 0.07 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, Y.; Jia, J.; Miao, H.; Ruan, W.; Wang, X. Performance Improvement and Biofouling Mitigation in Osmotic Microbial Fuel Cells via In Situ Formation of Silver Nanoparticles on Forward Osmosis Membrane. Membranes 2020, 10, 122. https://doi.org/10.3390/membranes10060122
Lu Y, Jia J, Miao H, Ruan W, Wang X. Performance Improvement and Biofouling Mitigation in Osmotic Microbial Fuel Cells via In Situ Formation of Silver Nanoparticles on Forward Osmosis Membrane. Membranes. 2020; 10(6):122. https://doi.org/10.3390/membranes10060122
Chicago/Turabian StyleLu, Yuqin, Jia Jia, Hengfeng Miao, Wenquan Ruan, and Xinhua Wang. 2020. "Performance Improvement and Biofouling Mitigation in Osmotic Microbial Fuel Cells via In Situ Formation of Silver Nanoparticles on Forward Osmosis Membrane" Membranes 10, no. 6: 122. https://doi.org/10.3390/membranes10060122