Purification of Methylsulfonylmethane from Mixtures Containing Salt by Conventional Electrodialysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
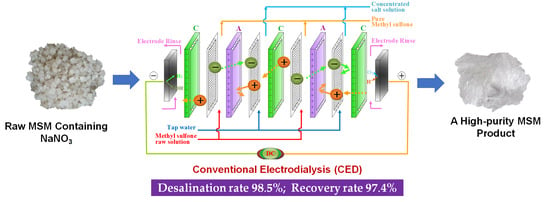
2.2. CED Set-Up
2.3. Analytical Methods
2.4. Calculation of Current Efficiency, Conversion Rate, and Energy Consumption
3. Results and Discussion
3.1. Effect of Operating Voltage
3.2. Effect of Feed MSM Concentration
3.3. Effect of the Electrolyte Salt Concentration
3.4. Process Economy
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Amirshahrokhi, K.; Bohlooli, S.; Chinifroush, M. The effect of methylsulfonylmethane on the experimental colitis in the rat. Toxicol. Appl. Pharmacol. 2011, 253, 197–202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pearson, T.W.; Dawson, H.J.; Lackey, H.B. Naturally occurring levels of dimethyl sulfoxide in selected fruits, vegetables, grains, and beverages. J. Agric. Food. Chem. 1981, 29, 1089–1091. [Google Scholar] [CrossRef] [PubMed]
- Sousa-Lima, I.; Park, S.-Y.; Chung, M.; Jung, H.J.; Kang, M.-C.; Gaspar, J.M.; Seo, J.A.; Macedo, M.P.; Park, K.S.; Mantzoros, C. Methylsulfonylmethane (MSM), an organosulfur compound, is effective against obesity-induced metabolic disorders in mice. Metabolism 2016, 65, 1508–1521. [Google Scholar] [CrossRef] [PubMed]
- Richmond, V.L. Incorporation of methylsulfonylmethane sulfur into guinea pig serum proteins. Life Sci. 1986, 39, 263–268. [Google Scholar] [CrossRef]
- Kim, L.; Axelrod, L.; Howard, P.; Buratovich, N.; Waters, R. Efficacy of methylsulfonylmethane (MSM) in osteoarthritis pain of the knee: A pilot clinical trial. Osteoarthr. Cartilage 2006, 14, 286–294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brien, S.; Prescott, P.; Bashir, N.; Lewith, H.; Lewith, G. Systematic review of the nutritional supplements dimethyl sulfoxide (DMSO) and methylsulfonylmethane (MSM) in the treatment of osteoarthritis. Osteoarthr. Cartilage 2008, 16, 1277–1288. [Google Scholar] [CrossRef] [Green Version]
- Ahn, H.; Kim, J.; Lee, M.-J.; Kim, Y.J.; Cho, Y.-W.; Lee, G.-S. Methylsulfonylmethane inhibits NLRP3 inflammasome activation. Cytokine 2015, 71, 223–231. [Google Scholar] [CrossRef]
- Wang, Y.M.; Zhang, Z.H.; Jiang, C.X.; Xu, T.W. Electrodialysis process for the recycling and concentrating of tetramethylammonium hydroxide (TMAH) from photoresist developer wastewater. Ind. Eng. Chem. Res. 2013, 52, 18356–18361. [Google Scholar] [CrossRef]
- Huang, C.H.; Xu, T.W.; Zhang, Y.P.; Xue, Y.H.; Chen, G.W. Application of electrodialysis to the production of organic acids: State-of-the-art and recent developments. J. Membr. Sci. 2007, 288, 1–12. [Google Scholar] [CrossRef]
- Mohammadi, T.; Razmi, A.; Sadrzadeh, M. Effect of operating parameters on Pb2+ separation from wastewater using electrodialysis. Desalination 2004, 167, 379–385. [Google Scholar] [CrossRef]
- Aktij, S.A.; Zirehpour, A.; Mollahosseini, A.; Taherzadeh, M.; Alberto, T.; Rahimpour, A. Feasibility of membrane processes for the recovery and purification of bio-based volatile fatty acids: A comprehensive review. J. Ind. Eng. Chem. 2020, 81, 24–40. [Google Scholar] [CrossRef]
- Wang, Y.M.; Zhang, Z.H.; Jiang, C.X.; Xu, T.W. Recovery of gamma-aminobutyric acid (GABA) from reaction mixtures containing salt by electrodialysis. Sep. Purif. Technol. 2016, 170, 353–359. [Google Scholar] [CrossRef]
- Wu, J.; Xu, C.Q.; Zhang, C.Y.; Wang, G.S.; Yan, Z.; Wu, C.M.; Wu, Y.H. Desalination of l-threonine (THR) fermentation broth by electrodialysis. Desalin. Water Treat. 2017, 81, 47–58. [Google Scholar] [CrossRef] [Green Version]
- Readi, O.M.K.; Rolevink, E.; Nijmeijer, K. Mixed matrix membranes for process intensification in electrodialysis of amino acids. J. Chem. Technol. Biotechnol. 2014, 89, 425–435. [Google Scholar] [CrossRef]
- Readi, O.M.K.; Girones, M.; Nijmeijer, K. Separation of complex mixtures of amino acids for biorefinery applications using electrodialysis. J. Membr. Sci. 2013, 429, 338–348. [Google Scholar] [CrossRef]
- Aghajanyan, A.E.; Hambardzumyan, A.A.; Vardanyan, A.A.; Saghiyan, A.S. Desalting of neutral amino acids fermentative solutions by electrodialysis with ion-exchange membranes. Desalination 2008, 228, 237–244. [Google Scholar] [CrossRef]
- Jiang, C.X.; Wang, Y.M.; Zhang, Z.H.; Xu, T.W. Electrodialysis of concentrated brine from RO plant to produce coarse salt and freshwater. J. Membr. Sci. 2014, 450, 323–330. [Google Scholar] [CrossRef]
- Liu, X.H.; Li, Q.H.; Jiang, C.X.; Lin, X.H.; Xu, T.W. Bipolar membrane electrodialysisin aqua-ethanol medium: Production of salicylic acid. J. Membr. Sci. 2015, 482, 76–82. [Google Scholar] [CrossRef]
- Buncel, E.; Nagelkerke, R.; Thatcher, G.R.J. Alkali metal ion catalysis in nucleophilic displacement by ethoxide ion on p-nitrophenyl phenylphosphonate: Evidence for multiple metal ion catalysis. Can. J. Chem. 2003, 81, 53–63. [Google Scholar] [CrossRef]
- Wang, X.L.; Wang, Y.M.; Zhang, X.; Feng, H.Y.; Xu, T.W. In-situ combination of fermentation and electrodialysis with bipolar membranes for the production of lactic acid: Continuous operation. Bioresour. Technol. 2013, 147, 442–448. [Google Scholar] [CrossRef]
- Li, J.; Morthensen, S.T.; Zhu, J.Y.; Yuan, S.S.; Wang, J.; Volodine, A.; Lin, J.Y.; Shen, J.N.; Van der Bruggen, B. Exfoliated MoS2 nanosheets loaded on bipolar exchange membranes interfaces as advanced catalysts for water dissociation. Sep. Purif. Technol. 2018, 194, 416–424. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.H.; Xu, T.W.; Jacobs, M.L. Regenerating flue-gas desulfurizing agents by bipolar membrane electrodialysis. AICHE J. 2006, 52, 393–401. [Google Scholar] [CrossRef]
- Strathmann, H.; Koops, G. Process economics of electrodialytic water dissociation for the production of acid and base. In Handbook on Bipolar Membrane Technology; Twente University Press Enschede: Enschede, The Netherlands, 2000; pp. 191–220. [Google Scholar]
- Wang, Y.M.; Wang, A.L.; Zhang, X.; Xu, T.W. Simulation of electrodialysis with bipolar membranes: estimation of process performance and energy consumption. Ind. Eng. Chem. Res. 2011, 50, 13911–13921. [Google Scholar] [CrossRef]












| Main Properties of Membranes | CJ-MC-2 | CJ-MA-2 |
|---|---|---|
| Thickness (mm) | 0.200 | 0.145 |
| Ion exchange capacity (mmol/g) | 1.50 | 1.25 |
| Water uptake (%) | 35 b | 32 b |
| Resistivity (Ω·cm2) | 2.0–3.5 c | 2.0–3.5 c |
| Transfer number (%) | 98 d | 99 d |
| Functional group | –SO3− | –N(CH3)3+ |
| Parameters | CED Process | Remarks |
|---|---|---|
| Feed solution volume (L) | 0.4 | |
| Experiment time (min) | 30 | |
| Voltage drop (V) | 10 | |
| Effective area of membrane (cm2) | 189 | |
| Electrolyte rinse solution, Na2SO4 (mol·L−1) | 0.3 | |
| Number of anion exchange membranes | 2 | |
| Number of cation exchange membranes | 3 | |
| Price of membrane ($·m−2) | 57.14 | |
| Energy consumption (kW·h·t−1) | 10 | |
| Treatment capacity (t·a−1) | 7.01 | 1 year 8760 h |
| Electricity charge ($·(kW·h)−1) | 0.086 | |
| Energy cost ($·t−1) | 0.86 | |
| Membranes cost ($·a−1) | 5.4 | |
| Stack cost ($·a−1) | 8.1 | ×1.5 membrane cost |
| Peripheral equipment cost ($·a−1) | 12.15 | ×1.5 stack cost |
| Total investment cost ($·a−1) | 20.25 | Stack cost + peripheral equipment cost |
| Amortization ($·a−1) | 6.75 | 3 years |
| Interest ($·a−1) | 1.62 | Interest rate, 8% |
| Maintenance ($·a−1) | 2.02 | 10% of total investment cost |
| Total fixed cost ($·a−1) | 10.39 | Amortization + interest + maintenance |
| Total fixed cost ($·t−1) | 1.48 | - |
| Total process cost ($·t−1) | 2.34 | Total fixed cost + energy cost |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, X.; Wang, Y.; Yan, H.; Wu, K.; Xu, T. Purification of Methylsulfonylmethane from Mixtures Containing Salt by Conventional Electrodialysis. Membranes 2020, 10, 23. https://doi.org/10.3390/membranes10020023
Wei X, Wang Y, Yan H, Wu K, Xu T. Purification of Methylsulfonylmethane from Mixtures Containing Salt by Conventional Electrodialysis. Membranes. 2020; 10(2):23. https://doi.org/10.3390/membranes10020023
Chicago/Turabian StyleWei, Xinlai, Yaoming Wang, Haiyang Yan, Ke Wu, and Tongwen Xu. 2020. "Purification of Methylsulfonylmethane from Mixtures Containing Salt by Conventional Electrodialysis" Membranes 10, no. 2: 23. https://doi.org/10.3390/membranes10020023