1. Introduction
Parkinson’s disease (PD) is a neurodegenerative disease characterized by the selective loss of dopaminergic (DA) neurons and the formation of Lewy bodies in the brain. The mainly symptoms was movement disorders such as bradykinesia, myotonia, tremors and abnormal gait [
1]. Many reasons such as apoptosis, oxidative stress, genetic factors, environmental factors, mitochondrial dysfunction, ubiquitin-proteasome system dysfunction, immune abnormalities, excitotoxicity and cytotoxicity of calcium may be related to the occurrence of PD [
2,
3]. Many studies have shown that DA neuron apoptosis has an important effect on the pathogenesis of PD, the number of apoptotic cells in PD patients is nearly 10 times more than normal aged person [
4]. However, the exact cause of DA neuron apoptosis is unknown [
5]. Oxidative stress, loss of antioxidant function and mitochondrial function damage, can induce DA neuron apoptosis at different levels [
6]. The inhibition of pro-apoptotic protein Bax and anti-apoptotic protein Bcl-2 can regulate the apoptosis of DA neurons [
7]. Therefore, regulation the expression of apoptosis-associated genes and proteins has become another strategy for the treatment of PD.
The PI3K–Akt pathway plays an important role in neuronal survival and death. Activation the PI3K–Akt pathway can inhibit the activity of downstream caspase-3 and thus inhibit the apoptosis of DA neurons, which can be weakened by the PI3K-specific inhibitor LY294002 [
8]. Studies found pretreatment with simvastatin, sulforaphane, erythropoietin, β-interferon and catechins on 6-OHDA-damaged SH-SY5Y cells can increase the PI3K phosphorylation and directly activates the PI3K signaling pathway. Activated Akt can inhibit the activity of downstream caspase-3 and thus inhibit the apoptosis of DA neurons, which can be weakened by the PI3K-specific inhibitor LY294002 [
9]. The brain tissues of PD patients and normal people were analyzed by immunofluorescence and western blotting after death. It was found that Akt and activated phosphoSer473-Akt were significantly reduced in the brains of PD patients. Nerve growth factor (NGF) plays an important role in the stages of neuron growth and development, axon growth, transmitter synthesis and cell apoptosis. Studies have shown that the application of corresponding treatments after spinal cord ischemia can induce NGF to activate the PI3K–Akt pathway to inhibit neuronal apoptosis. It can be seen that increase NGF to activate the PI3K–Akt pathway to inhibit DA neuronal apoptosis is a new strategy for the prevention and treatment of PD.
Marine seaweeds produce and accumulate a large number of substances with special chemical structures, physiological activities and functions during their growth and metabolism. The development and utilization of marine biologic resources is an important area and direction for drug candidates. The main feature of the polysaccharides extracted from seaweeds is that it contained sulfated groups. The structures were more complex and the activities were more excellent compared with the polysaccharides extracted from the land plants [
10]. Fucoidan is a kind of sulfated polysaccharide extracted from brown algae. It has various biologic activities such as antivirus, antitumor, antimutation, antiradiation and immunity enhancement [
11]. The bioactivity such as antioxidant, anticoagulation, neuro-protective activity of fucoidan depends on several structural parameters such as the degree of sulfation (DS), the molecular weight, other substitution groups and position, type of sugar and glycosidic branching. Our preliminary study had shown that the fucoidan with highest sulfated group exhibited stronger activity in scavenging superoxide radical and also hydroxyl radical [
12]. The sulfated and benzoylated derivatives of fucoidan could enhance the neuroprotective activity by increasing mitochondrial activity and decreasing LDH and ROS release induced by 6-OHDA (
p < 0.01 or
p < 0.001) [
13]. Our previous studies found that fucoidan (FPS) can reduce DA neurons damage in phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced PD mouse model [
14,
15]. FPS has a protective effects on oxidative damage and inflammatory lesion on DA neurons caused by MPTP in PD mouse, [
16]. FPS is a crude polysaccharide prepared from
Saccharina japonica. After degradation and purification, we got three fractions with different sulfated groups and monosaccharides compositions. Among the samples, UF with highest uronic acid and lowest sulfated groups has the strongest neuroprotective effect both in vitro and in vivo [
17]. UF can increase the level of antioxidant enzymes and reduce the level of lipid peroxidation in PD mice [
18]. We further found that UF can upregulate the expression of the anti-apoptotic protein Bcl-2, reduce the expression of the pro-apoptotic protein Bax, and significantly inhibit the apoptosis of DA neurons in H
2O
2–induced SH-SY5Y cell model [
13]. Therefore, we speculate that UF has effect on the DA neurons apoptosis. However, it is not yet established whether UF exhibited apoptosis activity through PI3K–Akt pathway in a MPP
+ induced neuronal cell line. Can UF activate PI3K by acting on the NGF to cause progressive activation of the PI3K–Akt pathway? How does UF regulate downstream signaling molecules and proteins in the PI3K–Akt pathway?
The aim of this study is to clarify whether UF can activate the PI3K–Akt pathway by acting on NGF protein and illuminate the anti-apoptosis mechanism of UF through the PI3K–Akt pathway. This study will provide experimental foundation and theoretical basis for the application of UF in PD therapy and provide a scientific basis for the development of new and effective anti-PD marine drugs.
3. Discussion
PD is a neurodegenerative disease caused by both genetic and environmental factors. Neuroprotection therapeutic method caused interest in recently. Previous study found fucoidan extracted from brown seaweeds had neuron protective activity which related to its antioxidant activity [
15]. Further studies found different fractions of fucoidan exhibited variety neuron protective activities. One fraction UF with higher uronic acid, lower sulfated group content and more complex monosaccharides exhibited the strongest activity. The neuron protective effect of UF may be mediated, in part, through antioxidant activity and the prevention of cell apoptosis [
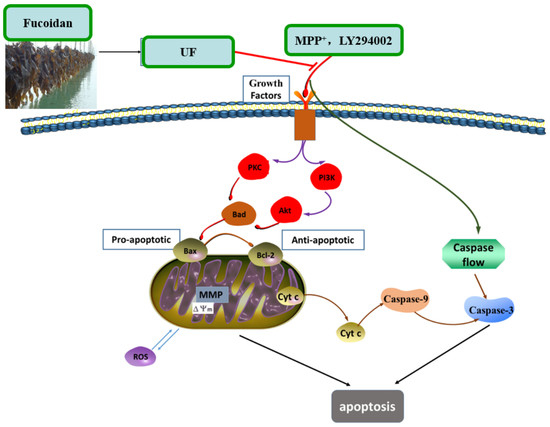
18]. The simplified depiction effect of UF on MPP
+-induced cytotoxicity was summarized in
Figure 7. UF treatment groups could decrease the cell apoptosis rate and increase the cell vitality. LY294002 could inactivate the PI3K–Akt pathway, thereby inhibiting cell proliferation and inducing apoptosis. The results found that the neuron protective effect of UF was alleviated when treated with LY294002. It is illustrated that the neuron protective effect of UF may relate with the PI3K–Akt pathway.
The PI3K–Akt pathway plays an important role in the survival and maintenance of many neuronal function such as long-term potentiation and memory formation. Inhibition of PI3K activity can offset the ability of nerve growth factors to promote cell survival [
8]. Chondroitin sulfate (CS) in the cell matrix can protect SH-SY5Y cells by activating the PI3K–Akt signaling pathway [
6]. Nerve growth factor (NGF) is a peptide molecule that plays a nutritional role in nerve cells. In the nervous system, NGF can increase the tolerance of cells under oxidative stress, which is the main mechanism involves NGF-induced activation of the PI3K–Akt pathway. Studies have shown that the application of corresponding treatments after spinal cord ischemia can induce NGF to activate the PI3K–Akt pathway to inhibit neuronal apoptosis [
20]. Seow et al. found crude polysaccharides extracted from
Lignosus rhinocerotis could stimulate neurogenesis without stimulating the production of NGF in PC-12 cells [
21]. We found UF had a backbone of alternating 4-linked GlcA and 2-linked Man with the first Man residue from the nonreducing end accidentally sulfated at C6. UF and CS both have GluA and sulfate group, so we suppose UF could combine with NGF and increase the expression of the NGF, then activation the PI3K–Akt pathway. Our study confirmed that UF treatment could increase the expression of NGF, the effect was alleviated when added LY294002, but not disappeared completely. From our data, we suppose UF exhibited protective effect against MPP
+-induced SH-SY5Y cell apoptosis by activating PI3K–Akt pathway through reacting with NGF. The chemical composition and structure of the polysaccharide had relationship with the effect on the NGF, chondroitin sulfate and fucoidan could increase the expression of the NGF protein, however, polysaccharide extracted from
Lignosus rhinocerotis mimics the neurogenic activity of NGF.
PI3K is one of the signal molecules involved in intracellular signal transduction. When cells are stimulated by stimulating factors such as NGF, the phosphorylation of PI3K is activated. The present results demonstrated that UF treatment could enhance the phosphorylation of PI3K first, and then promoted the phosphorylation of Akt. This effect was alleviated when combined with LY294002.
When phosphorylating the PI3K and Akt, it activates or inhibits its downstream target proteins Bad, Bcl-2, Bax, caspase-9, GSK-3, mTOR, nuclear transcription factors, etc., in turn regulate cell proliferation, differentiation, apoptosis and invasion [
22]. The influence of UF on these anti-apoptotic and pro-apoptotic protein were analyzed. Our study showed that UF could partially inhibit MPP
+-induced dysfunction of the Bax/Bcl-2 system and decrease the expression of P53 and GSK3β protein, then inhibited cell apoptosis. This impact was alleviated when adding LY294002 in UF treated groups. As an anti-apoptotic member of the Bcl-2 family, Bcl-2 can bind Bax to form Bax/Bcl-2 heterodimers, thereby, attenuating the pro-apoptotic effect of Bax. Habaike
et al. found polysaccharides extracted from
Laricifomes officinalis Ames could attenuating cell apoptosis, increasing the ratio of Bcl-2/Bax and inhibiting cytochrome C release from mitochondria to cytosol in PC12 cells [
23]. An acid-soluble polysaccharide (GFAP) prepared from
Grifola frondosa could upregulate the expressions of Bax in HCC cells and induced the cell apoptosis [
24]. The UF is a heteropolysaccharide with uronic acid and sulfate group, we suppose it can react with Bax and effect the formation of Bax/Bcl-2 heterodimers. P53 and GSK3β are thought to be a key factor in the subsequent apoptotic processes among the pro-apoptotic proteins. UF can react with these two proteins directly and reduce the cell apoptosis in the H
2O
2-reduced cell model [
25].
Caspase family such as caspase-3 (cas3), caspase-8 (cas8) and caspase-9 (cas9) are crucial checkpoint in cell commitment to apoptosis. Cas3 is a critical executor of apoptosis being responsible for the proteolytic cleavage of many key proteins, which damage initiates the cell death program. Yu et al. found an acid-soluble polysaccharide could trigger apoptosis of HCC cells through mitochondria apoptotic pathway in a caspases-dependent pattern [
24]. It means the acid polysaccharide could react with caspases-dependent pattern, it could decrease or increase the expression of caspase protein, which confirmed by our results. The addition of UF significantly attenuated the expression of cas3 in MPP
+-induced SH-SY5Y cells, confirming UF had effect in terms of apoptosis process. LY294002 used did not lead to a complete inhibition of initiation-programmed cell death by UF treated groups, suggesting that other pathways are also involved. Cas8 and cas9 have shown increase in MPP
+ and MPP
++LY294002 groups simultaneously, UF treated groups could decrease this rising tendency. The observed changes may suggest that UF could significantly decrease cas3, cas8 and casp9 activation, the consequence of which is apoptosis.
Our study showed that UF treatment could reversed the toxic effect of MPP+ on SH-SY5Y cells by activation the PI3K–Akt pathway. Addition of LY294002 significantly inhibited the PI3K–Akt pathway active and enhanced DNA fragmentation in SH-SY5Y cells, UF treated groups could still alleviate the cell apoptosis. In the present study, we demonstrated for the first time that UF attenuated the MPP+ induced apoptosis via reacting with NGF, Bax and cas3. Our data indicated that UF inhibited cell apoptosis by participating in PI3K–Akt pathway partially.
4. Materials and Methods
4.1. Preparation of Sulfated Polysaccharides
Saccharina japonica (Laminariaceae), cultured along the coast of Dandong, China, was collected in August 2015, authenticated by Prof. Lanping Ding and stored as a voucher specimen (No. 83) in the Institute of Oceanology, CAS. The fresh algae were promptly washed, sun dried and kept in plastic bags at room temperature until use. FPS and UF were prepared according to our previous methods with minor modifications (
Figure 8) [
12,
26]. Briefly, FPS was extracted in water solution at 120 ˚C using autoclave from
Saccharina japonica. DF was prepared using ascorbate and hydrogen peroxide (30 mmol/L, 1:1) degradation method. UF were obtained using DEAE-Sepharose FF exchange chromatography previously described.
4.2. Analytical Methods
The total sugar content of samples were determined according to the method of Dubois et al. using L-fucose as the standard [
27]. The sulfate content was analyzed by ion chromatography using K
2SO
4 as the standard. Uronic acid was estimated via a modified carbazole method using
d-glucuronic acid as the standard [
28]. The neutral sugar composition was determined by PMP-HPLC precolumn derivatization chromatography using ribose as interior label [
29]. The molecular weight was assayed by an HP-GPC chromatography using a series of dextrans with different molecular weights as standards. A series of dextrans were purchased from the National Institute for the Control of Pharmaceutical and Biologic Products (China). Other standard reagents were purchased from Sigma-Aldrich (Milwaukee, WI, USA).
4.3. Cell Culture and Treatments
A dopaminergic cell line SH-SY5Y was used to establish an in vitro PD model. SH-SY5Ycells were kindly provided by Professor Ning Song (QingDao University) and maintained in Dulbecco’s modified Eagle medium/F12 supplemented with 10% newborn calf serum (Gibco) in an incubator with an atmosphere of 5% CO2 at 37 °C. For all experiments, the cells were seeded on 96-well plates, 24-well plates or 6-well plates at a density of 1 × 104 cells–1 × 105 cells/mL for 24 h. Then the cells were incubated with MPP+ for 30 min, then treated with different reagents for 24 h. The cells were divided into 12 groups. 1. NC group: treated with DMEM; 2: MPP group: treated with 100-µM MPP+; 3. UF1 group: treated with 100-µM MPP+ and 100-µg/mL UF; 4. UF2 group: treated with 100-µM MPP+ and 500-µg/mL UF; 5. UF3 group: treated with 100-µM MPP+ and 800-µg/mL UF; 6. MA group: treated with 100-µM MPP+ and 100-mM positive drugs Modopar; 7. NCLY group: treated with DMEM and 20-µM LY294002; 8: MPPLY group: treated with 100-µM MPP+ and 20-µM LY294002; 9. UF1LY group: treated with 100-µM MPP+, 100-µg/mL UF and 20-µM LY294002; 10. UF2LY group: treated with 100-µM MPP+, 500-µg/mL UF and 20-µM LY294002; 11. UF3LY group: treated with 100-µM MPP+, 800-µg/mL UF and 20-µM LY294002; 12. MALY group: treated with 100-µM MPP+, 100-mM positive drugs Modopar and 20-µM LY294002. All the group had six wells and all the experiments were repeated three times in different batches of cells.
4.4. Measurement of Cell Viability by MTT
SH-SY5Y cells were plated at a density of 1 × 10
4 cells/100 µL in 96-well plates. Cell viability was quantitatively assessed using the MTT ([3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl tetrazolium bromide]) assay [
9]. After 24 h treatment, 20 µL MTT (0.5 mg/mL) regent was added to each well and incubated at 37 °C for 4 h. The medium was removed and washed twice with phosphate buffer solution (pH 7.4), then 200 µL DMSO was added to solubilize the formazan crystals. Cell viability was measured at 494 nm by spectrophotometer (Bio-Tec Gen 5, Winooski, VT, USA). Unless stated otherwise, all other chemicals were purchased from Sigma-Aldrich.
4.5. Observation of Morphologic Changes
Cells were seeded in 24-well plates at a density of 1 × 105 for 24 h. After treatment for 24 h, cells were washed with phosphate-buffered saline and stained with 400 µL Hoechst 33,342 (2.5 µg/mL) for 5 min in the dark. After removing the medium and washed twice with phosphate buffer solution (pH 7.4), 400 µL PI (12.5 µg/mL) was added for 5 min in the dark. Cells with typical apoptotic nuclear morphology such as nuclear shrinkage and fragmentation and micronuclei formation were identified under fluorescent microscope and counted using randomly selected fields on numbered slides. The percentage of apoptotic cells was scored by counting at least 200 cells per treatment group and the average percentage of apoptotic cells was determined for each UF treatment and expressed as the mean ± SD.
4.6. Immunocytochemistry
Immunocytochemistry was performed and modified according to Iida’s study. Briefly, SH-SY5Y cells were seeded in 12-wells plates and incubation with MPP+ and different reagent for 24 h. After washing with PBS for three times and fixing with PBS containing 4% (wt/vol) paraformaldehyde for 15 min and then permeabilized with 0.5% (wt/vol) Triton X-100 and blocked with 5% goat serum for 1 h at room temperature. Subsequently, the cells were incubated with rabbit monoclonal anti-Akt (1:200), anti-PI3K (1:200), anti-Bax (1:100), anti-Bcl-2 (1:100), anti-GSK3β (1:200), anti-pPI3K (1:200), anti-p53 (1:100), anti-NGF (1:200), anti-pAkt (1:200) antibodies at 4 °C overnight. After washing with PBST and incubated with the second antibody (1:200) in PBST for 1 h. After the samples were washed with PBS three times, they were embedded in DAPI for 5 min and then washed with PBST 4 times. The images were obtained using an Olyba microscope. The mean fluorescence intensity was calculated by Image-Pro (Rockville, MD, USA).
4.7. Western Blot Analysis
Cells were lysed in lysis solution (Ambion, Grand Island, NY, USA) and incubated at 95 °C for 10 min. Protein concentration was determined by the Bradford assay kit (Takara Biotechnology, Dalian, China). Twenty micrograms of total proteins was separated by 10%–12% sodium dodecyl sulfate polyacrylamide gels and then transferred to polyvinylidene difluoride membranes. Blots were probed with rabbit monoclonal anti-Akt (1:1000), anti-pAkt (1:1000), anti-PI3K (1:1000), anti-pPI3K (1:1000). Blots were also probed with rabbit monoclonal anti-GAPDH antibody (Milwaukee, WI, USA, Sigma, 1:10,000) as a loading control. Anti-rabbit secondary antibodies conjugated to horseradish peroxidase were used at 1:10,000 (Santa Cruz Biotechnology, Santa Cruz, CA, USA). UVP BioSpectrum®CCD imaging system (Davis, CA, USA) was used for imaging and analysis. Camera settings were manipulated in preview mode to optimize the exposure and determine the appropriate final exposure settings. Exposures of 30 s up to 5 min were used for data collection. Results were analyzed through scanning densitometry by UVP Vision Works LS Software (UVP, Cambridge, UK).
4.8. Total RNA Extraction and Real Time PCR
SH-SY5Y Cells were seeded in 6-wells plates and incubation with MPP
+ and different reagent for 24 h. Total RNA was isolated by Trizol Reagent (Takara Biotechnology, Dalian, China) ccording to the manufacturer’s instructions. From each sample, 1 µg of total RNA was retrotranscripted into cDNA (Takara RR047A, Dalian, China). Then, 2 µL of each sample was used as a template for amplification reactions conducted with the SYBR Premix Ex TaqTMⅡ (Takara Biotechnology, Dalian, China) following the manufacturer’s instructions. The PCR amplifications were conducted using a life Technology 7500 fast Real-time PCR system. The expression of house-keeping gene, GAPDH mRNA, was served as the standardized control. Primer (showed in
Table 2) selection was performed using the Primer Premier Design Software, version 1.0 (Idaho Technology, Inc., Alameda, CA, USA). The mRNA level for the control group was set as 100%.
4.9. Caspase-3, -8 and -9 Activity
After treatment of cells with UF for 24 h, the cells were harvested using cell scrapers and washed in ice-cold PBS. Then, the cells were lysed for 30 min on the ice in 100 µL of Cell Lysis Reagent supplemented with complete protease inhibitor cocktail. The protein concentration of cell lysates was determined by Bicinchoninic acid (BCA) assay (Takara Biotechnology, Dalian, China).
4.10. Statistical Analysis of Data
The data are presented as the mean values ± 1 SD (n = 8−10). The data were analyzed by a one-way ANOVA, a Duncan’s multiple-range test and an LSD test at a significance level of p < 0.05. SPSS 22.0 software (New York, NY, USA) was used for the analysis.