Acylated Aminooligosaccharides with Inhibitory Effects against α-Amylase from Streptomyces sp. HO1518
Abstract
:1. Introduction
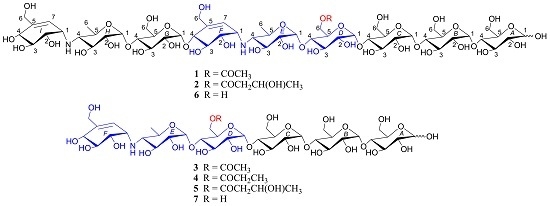
2. Results and Discussion
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Strain Isolation and Cultivation
3.3. Extraction and Isolation
3.4. Conversion of Compounds 1 and 2 to 6 and 3–5 to 7
3.5. Biological Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cantarel, B.L.; Coutinho, P.M.; Rancurel, C.; Bernard, T.; Lombard, V.; Henrissat, B. The carbohydrate-active enzymes database (cazy): An expert resource for glycogenomics. Nucleic. Acids Res. 2009, 37, D233–D238. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.H.; Robyt, J.F. Study of the inhibition of four α-amylases by acarbose and its 4IV-α-maltohexaosyl and 4IV-α-maltododecaosyl analogues. Carbohydr. Res. 2003, 338, 1969–1980. [Google Scholar] [CrossRef]
- De Sales, P.M.; de Souza, P.M.; Dartora, M.; Resck, I.S.; Simeoni, L.A.; Fonseca-Bazzo, Y.M.; de Oliveira Magalhães, P.; Silveira, D. Pouteria torta epicarp as a useful source of α-amylase inhibitor in the control of type 2 diabetes. Food Chem. Toxicol. 2017, 109, 962–969. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, D.D.; Frommer, W.; Junge, B.; Müller, L.; Wingender, W.; Truscheit, E.; Schäfer, D. α-Glucosidase inhibitors. New complex oligosaccharides of microbial origin. Naturwissenschaften 1977, 64, 535–536. [Google Scholar] [CrossRef] [PubMed]
- Truscheit, E.; Frommer, W.; Junge, B.; Müller, L.; Schmidt, D.D.; Wingender, W. Chemistry and biochemistry of α-glucosidase inhibitors. Angew. Chem. Int. Ed. Engl. 1981, 20, 744–761. [Google Scholar] [CrossRef]
- Yokose, K.; Ogawa, K.; Sano, T.; Watanabe, K.; Maruyama, H.B.; Suhara, Y. New α-amylase inhibitor, trestatins I. Isolation characterization and biological activities of trestatins A, B, and C. J. Antibiot. 1983, 36, 1157–1165. [Google Scholar] [CrossRef] [PubMed]
- Yokose, K.; Ogawa, K.; Suzuki, Y.; Umeda, I.; Suhara, Y. New α-amylase inhibitor, trestatins II. Structure determination of trestatins A, B and C. J. Antibiot. 1983, 36, 1166–1175. [Google Scholar] [CrossRef] [PubMed]
- Shi, D.-Y.; Zhong, D.-F.; Chen, X.-Y. Profiling of isovalertatin family aminooligosaccharides extracted from the culture of Streptomyces luteogriseus by using liquid chromatography/electrospray ionization mass spectrometry. Anal. Chem. 2001, 73, 3808–3815. [Google Scholar]
- Zhong, D.; Si, D.-Y.; He, W.-Y.; Zhao, L.-M.; Xu, Q.-M. Structural revision of isovalertatins M03, M13, and M23 isolated from the culture of Streptomyces luteogriseus. Carbohydr. Res. 2001, 331, 69–75. [Google Scholar] [CrossRef]
- Si, D.-Y.; Zhong, D.-F.; Xu, Q.-M. Two butylated aminooligosaccharides isolated from the culture filtrate of Streptomyces luteogriseus. Carbohydr. Res. 2001, 335, 127–132. [Google Scholar] [CrossRef]
- Geng, P.; Qiu, F.; Zhu, Y.-Y.; Bai, G. Four acarviosin-containing oligosaccharides identified from Streptomyces coelicoflavus ZG0656 are potent inhibitors of α-amylase. Carbohydr. Res. 2008, 343, 882–892. [Google Scholar] [CrossRef] [PubMed]
- Geng, P.; Bai, G. Two novel aminooligosaccharides isolated from the culture of Streptomyces coelicoflavus ZG0656 as potent inhibitors of α-amylase. Carbohydr. Res. 2008, 343, 470–476. [Google Scholar] [CrossRef] [PubMed]
- Geng, P.; Meng, X.-S.; Bai, G.; Luo, G.-A. Profiling of acarviostatin family secondary metabolites secreted by Streptomyces coelicoflavus ZG0656 using ultraperformance liquid chromatography coupled with electrospray ionization mass spectrometry. Anal. Chem. 2008, 80, 7554–7561. [Google Scholar] [CrossRef] [PubMed]
- Geng, P.; Sun, T.; Zhong, Q.-P.; Li, X.-X.; Shi, L.-Y.; Bai, F.; Bai, G. Two novel potent α-amylase inhibitors from the family of acarviostatins isolated from the culture of Streptomyces coelicoflavus ZG0656. Chem. Biodivers. 2013, 10, 452–459. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.-Q.; Cui, Q.-X.; Hou, Y.-Y.; Bai, F.; Sun, J.-X.; Cao, X.-F.; Liu, P.; Jiang, M.; Bai, G. An integrated strategy of ultra-high-performance liquid chromatography/quadrupole-time-of-flight mass spectrometry and virtual screening for the identification of α-glucosidase inhibitors in acarviostatin-containing complex. J. Chromatogr. A 2013, 1319, 88–96. [Google Scholar] [CrossRef]
- Meng, P.; Guo, Y.-Q.; Zhang, Q.; Hou, J.; Bai, F.; Geng, P.; Bai, G. A novel amino-oligosaccharide isolated from the culture of Streptomyces strain PW638 is a potent inhibitor of α-amylase. Carbohydr Res. 2011, 346, 1898–1902. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.-H.; Ren, L.-M.; Yang, X.; Bai, F.; Wang, L.-L.; Geng, P.; Bai, G.; Shen, Y.-Q. Structures of human pancreatic α-amylase in complex with acarviostatins: Implications for drug design against type II diabetes. J. Struct. Biol. 2011, 174, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Geng, P.; Bai, G.; Shi, Q.; Zhang, L.; Gao, Z.-H.; Zhang, Q. Taxonomy of the Streptomyces strain ZG0656 that produces acarviostatin α-amylase inhibitors and analysis of their effects on blood glucose levels in mammalian systems. J. Appl. Microbiol. 2009, 106, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Z.-Q.; Liu, Q.-X.; Pan, Z.-L.; Zhao, N.; Feng, Z.-X.; Wang, Y. Diversity and bioprospecting of culturable actinomycetes from marine sediment of the Yellow Sea, China. Arch. Microbiol. 2015, 197, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Z.-Q.; Wang, J.-F.; Hao, Y.-Y.; Wang, Y. Recent advances in marine microbial natural products discovery and development. Mar. Drugs 2013, 11, 700–717. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Z.-Q.; Wang, Y. Draft genome sequence of the marine Streptomyces sp. strain AA1529, isolated from the Yellow Sea. J. Bacteriol. 2012, 194, 5474–5475. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Z.-Q.; Wang, Y. Draft genome sequence of marine-derived Streptomyces sp. strain AA0539, isolated from the Yellow Sea, China. J. Bacteriol. 2012, 194, 6622–6623. [Google Scholar] [CrossRef] [PubMed]
- Ono, M.; Oda, S.; Yasuda, S.; Mineno, T.; Okawa, M.; Kinjo, J.; Miyashita, H.; Yoshimitsu, H.; Nohara, T.; Miyahara, K. Acylated glycosidic acid methyl ester generated from the convolvulin fraction of Rhizoma Jalapae Braziliensis by treatment with indium (III) chloride in methanol. Chem. Pharm. Bull. 2017, 65, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Trujillo, M.E.; Miyanaga, S.; Saiki, I.; Igarashi, Y. Campechic acids A and B: anti-invasive polyether polyketides from a soil-derived Streptomyces. J. Nat. Prod. 2014, 77, 976–982. [Google Scholar] [CrossRef] [PubMed]







| No. | 1 c | 2 c | 6 | |||
|---|---|---|---|---|---|---|
| δC, Type | δH (J in Hz) | δC, Type | δH (J in Hz) | δC, Type | δH (J in Hz) | |
| A1α | 94.8, CH | 5.23, d (3.5) | 94.8, CH | 5.23, d (3.5) | 94.8, CH | 5.23, d (3.5) |
| A2α | 74.2, CH | 3.57, m | 74.2, CH | 3.57, m | 74.1, CH | 3.57, m |
| A3α | 76.1, CH | 3.98, m | 76.0, CH | 3.98, m | 76.0, CH | 3.98, m |
| A4α | 79.6, CH | 3.66, m | 79.6, CH | 3.66, m | 79.6, CH | 3.66, m |
| A5α | 72.8, CH | 3.94, m | 72.8, CH | 3.94, m | 72.8, CH | 3.94, m |
| A6α | 63.3, CH2 | 3.84, m | 63.3, CH2 | 3.84, m | 63.3, CH2 | 3.84, m |
| A1β | 98.6, CH | 4.66, d (8.0) | 98.7, CH | 4.66, d (8.0) | 98.6, CH | 4.66, d (8.0) |
| A2β | 76.9, CH | 3.28, dd (9.5, 8.0) | 76.7, CH | 3.28, dd (9.5, 8.0) | 76.8, CH | 3.28, dd (9.5, 8.0) |
| A3β | 79.1, CH | 3.78, m | 79.1, CH | 3.77, m | 79.0, CH | 3.77, m |
| A4β | 79.7, CH | 3.66, m | 79.7, CH | 3.66, m | 79.7, CH | 3.66, m |
| A5β | 77.4, CH | 3.60, m | 77.4, CH | 3.60, m | 77.4, CH | 3.60, m |
| A6β | 63.3, CH2 | 3.91, m | 63.4, CH2 | 3.91, m | 63.3, CH2 | 3.91, m |
| B1 | 102.5, CH | 5.41, d (3.5) | 102.5, CH | 5.41, d (3.5) | 102.5, CH | 5.41, d (3.5) |
| B2 | 74.4, CH | 3.63, m | 74.4, CH | 3.63, m | 74.4, CH | 3.63, m |
| B3 | 76.2, CH | 3.96, m | 76.2, CH | 3.96, m | 76.2, CH | 3.96, m |
| B4 | 79.9, CH | 3.67, m | 79.9, CH | 3.67, m | 79.9, CH | 3.67, m |
| B5 | 74.0, CH | 3.85, m | 74.0, CH | 3.85, m | 74.0, CH | 3.85, m |
| B6 | 63.4, CH2 | 3.84, m | 63.4, CH2 | 3.84, m | 63.4, CH2 | 3.84, m |
| C1 | 102.5, CH | 5.41, d (3.5) | 102.5, CH | 5.41, d (3.5) | 102.5, CH | 5.41, d (3.5) |
| C2 | 74.4, CH | 3.63, m | 74.4, CH | 3.63, m | 74.4, CH | 3.63, m |
| C3 | 76.2, CH | 3.96, m | 76.2, CH | 3.96, m | 76.2, CH | 3.96, m |
| C4 | 79.9, CH | 3.67, m | 79.9, CH | 3.67, m | 79.9, CH | 3.67, m |
| C5 | 74.0, CH | 3.85, m | 74.0, CH | 3.85, m | 74.0, CH | 3.85, m |
| C6 | 63.4, CH2 | 3.84, m | 63.5, CH2 | 3.84, m | 63.4, CH2 | 3.84, m |
| D1 | 102.5, CH | 5.41, d (3.5) | 102.5, CH | 5.40, d (3.5) | 102.5, CH | 5.41, d (3.5) |
| D2 | 74.4, CH | 3.66, m | 74.4, CH | 3.66, m | 74.4, CH | 3.66, m |
| D3 | 76.2, CH | 3.95, m | 76.2, CH | 3.96, m | 76.2, CH | 3.96, m |
| D4 | 80.7, CH | 3.66, m | 80.8, CH | 3.66, m | 79.8, CH | 3.66, m |
| D5 | 71.7, CH | 4.06, m | 71.8, CH | 4.07, m | 74.0, CH | 3.85, m |
| D6a | 66.5, CH2 | 4.43, dd (12.5, 2.0) | 66.5, CH2 | 4.50, dd (12.5, 2.0) | 63.5, CH2 | 3.84, m |
| D6b | 4.24, m | 4.26, m | ||||
| E1 | 103.4, CH | 5.30, d (3.5) | 103.4, CH | 5.29, d (3.5) | 102.7, CH | 5.33, d (3.5) |
| E2 | 74.1, CH | 3.59, m | 74.1, CH | 3.60, m | 74.1, CH | 3.60, m |
| E3 | 75.8, CH | 3.63, m | 75.8, CH | 3.63, m | 75.8, CH | 3.63, m |
| E4 | 67.1, CH | 2.46, t (9.0) | 67.1, CH | 2.47, t (9.0) | 67.0, CH | 2.47, t (9.0) |
| E5 | 72.6, CH | 3.74, m | 72.6, CH | 3.76, m | 72.5, CH | 3.74, m |
| E6 | 20.2, CH3 | 1.31, d (6.0) | 20.2, CH3 | 1.32, d (6.0) | 20.2, CH3 | 1.32, d (6.0) |
| F1 | 57.8, CH | 3.53, m | 57.9, CH | 3.53, m | 57.9, CH | 3.53, m |
| F2 | 72.5, CH | 3.80, m | 72.5, CH | 3.80, m | 72.4, CH | 3.80, m |
| F3 | 73.6, CH | 4.16, m | 73.7, CH | 4.16, m | 73.5, CH | 4.16, m |
| F4 | 79.1, CH | 4.24, d (6.4) | 79.1, CH | 4.23, d (6.4) | 78.9, CH | 4.23, d (6.4) |
| F5 | 139.4, C | 139.3, C | 139.3, C | |||
| F6a | 64.9, CH2 | 4.22, m | 64.9, CH2 | 4.22, m | 64.9, CH2 | 4.22, m |
| F6b | 4.14, m | 4.14, m | 4.14, m | |||
| F7 | 129.2, CH | 5.98, d (4.5) | 129.2, CH | 5.98, d (4.5) | 129.1, CH | 5.98, d (4.5) |
| G1 | 100.4, CH | 5.38, d (4.0) | 100.4, CH | 5.38, d (4.0) | 100.4, CH | 5.38, d (4.0) |
| G2 | 74.3, CH | 3.63, m | 74.3, CH | 3.63, m | 74.4, CH | 3.63, m |
| G3 | 76.4, CH | 3.92, m | 76.4, CH | 3.92, m | 76.3, CH | 3.92, m |
| G4 | 79.9, CH | 3.61, m | 79.9, CH | 3.61, m | 79.9, CH | 3.61, m |
| G5 | 74.0, CH | 3.90, m | 74.0, CH | 3.90, m | 74.0, CH | 3.90, m |
| G6 | 63.5, CH2 | 3.86, m | 63.6, CH2 | 3.86, m | 63.5, CH2 | 3.86, m |
| H1 | 102.8, CH | 5.33, d (3.5) | 102.8, CH | 5.34, d (3.5) | 102.7, CH | 5.33, d (3.5) |
| H2 | 74.2, CH | 3.58, m | 74.2, CH | 3.59, m | 74.1, CH | 3.58, m |
| H3 | 75.4, CH | 3.61, m | 75.4, CH | 3.63, m | 75.4, CH | 3.61, m |
| H4 | 67.8, CH | 2.48, t (9.0) | 67.8, CH | 2.49, t (9.0) | 67.8, CH | 2.48, t (9.0) |
| H5 | 72.5, CH | 3.76, m | 72.5, CH | 3.78, m | 72.3, CH | 3.76, m |
| H6 | 20.2, CH3 | 1.35, d (6.0) | 20.2, CH3 | 1.36, d (6.0) | 20.2, CH3 | 1.35, d (6.0) |
| I1 | 58.8, CH | 3.54, m | 58.9, CH | 3.54, m | 58.8, CH | 3.54, m |
| I2 | 75.5, CH | 3.67, m | 75.5, CH | 3.67, m | 75.5, CH | 3.67, m |
| I3 | 75.6, CH | 3.77, m | 75.6, CH | 3.77, m | 75.6, CH | 3.77, m |
| I4 | 73.8, CH | 4.05, d (6.4) | 73.8, CH | 4.05, d (6.4) | 73.7, CH | 4.05, d (6.4) |
| I5 | 141.8, C | 141.8, C | 141.8, C | |||
| I6a | 64.5, CH2 | 4.23, m | 64.5, CH2 | 4.24, m | 64.4, CH2 | 4.23, m |
| I6b | 4.12, m | 4.12, m | 4.12, m | |||
| I7 | 126.6, CH | 5.90, dd (5.0, 1.0) | 126.7, CH | 5.91, dd (5.0, 1.0) | 126.6, CH | 5.91, dd (5.0, 1.0) |
| D6-O-acetyl | 177.0, C | - | - | - | - | |
| 23.1, CH3 | 2.17 (3H, s) | - | - | - | - | |
| D6-O-2-hydroxybutyryl | - | - | 176.6, C | - | - | |
| - | - | 45.9, CH2 | 2.67, dd (15.0, 4.5) 2.59, dd (15.0, 8.0) | - | - | |
| - | - | 67.3, CH | 4.27, m | - | - | |
| - | - | 24.8, CH3 | 1.26, d (6.5) | - | - | |
| No. | 3 a,c | 4 a,c | 5 b,c | 7 [11] | |||
|---|---|---|---|---|---|---|---|
| δC, Type | δH (J in Hz) | δC, Type | δH (J in Hz) | δC, Type | δH (J in Hz) | δC, Type | |
| A1α | 94.7, CH | 5.23, d (3.5) | 94.8, CH | 5.23, d (3.5) | 94.8, CH | 5.24, d (3.5) | 93.0 |
| A2α | 74.2, CH | 3.58, m | 74.1, CH | 3.58, m | 74.2, CH | 3.58, m | 72.6 |
| A3α | 76.0, CH | 3.98, m | 76.0, CH | 3.98, m | 76.0, CH | 3.98, m | 74.4 |
| A4α | 79.5, CH | 3.66, m | 79.6, CH | 3.66, m | 79.5, CH | 3.67, m | 79.3 |
| A5α | 72.8, CH | 3.94, m | 72.8, CH | 3.94, m | 72.8, CH | 3.95, m | 71.0 |
| A6α | 63.3, CH2 | 3.84, m | 63.2, CH2 | 3.84, m | 63.2, CH2 | 3.84, m | 61.5 |
| A1β | 98.6, CH | 4.66, d (8.0) | 98.6, CH | 4.66, d (8.0) | 98.6, CH | 4.66, d (8.0) | 96.8 |
| A2β | 76.9, CH | 3.28, dd (9.5, 8.0) | 76.9, CH | 3.28, dd (9.5, 8.0) | 76.9, CH | 3.28, dd (9.5, 8.0) | 75.1 |
| A3β | 79.1, CH | 3.77, m | 79.0, CH | 3.78, m | 79.1, CH | 3.78, m | 77.3 |
| A4β | 79.6, CH | 3.66, m | 79.7, CH | 3.66, m | 79.6, CH | 3.66, m | 77.8 |
| A5β | 77.4, CH | 3.60, m | 77.4, CH | 3.60, m | 77.4, CH | 3.60, m | 75.6 |
| A6β | 63.5, CH2 | 3.91, m | 63.5, CH2 | 3.91, m | 63.5, CH2 | 3.91, m | 61.5 |
| B1 | 102.5, CH | 5.41, d (3.5) | 102.5, CH | 5.41, d (3.5) | 102.5, CH | 5.41, d (3.5) | 100.7 |
| B2 | 74.4, CH | 3.63, m | 74.4, CH | 3.63, m | 74.4, CH | 3.63, m | 72.6 |
| B3 | 76.2, CH | 3.96, m | 76.2, CH | 3.96, m | 76.2, CH | 3.96, m | 74.4 |
| B4 | 79.8, CH | 3.67, m | 79.9, CH | 3.67, m | 79.8, CH | 3.67, m | 78.0 |
| B5 | 74.0, CH | 3.85, m | 74.0, CH | 3.85, m | 74.0, CH | 3.85, m | 72.2 |
| B6 | 63.4, CH2 | 3.84, m | 63.2, CH2 | 3.84, m | 63.4, CH2 | 3.84, m | 61.5 |
| C1 | 102.5, CH | 5.41, d (3.5) | 102.5, CH | 5.41, d (3.5) | 102.5, CH | 5.41, d (3.5) | 100.7 |
| C2 | 74.4, CH | 3.63, m | 74.4, CH | 3.63, m | 74.4, CH | 3.63, m | 72.6 |
| C3 | 76.2, CH | 3.96, m | 76.2, CH | 3.96, m | 76.2, CH | 3.96, m | 74.4 |
| C4 | 79.8, CH | 3.67, m | 79.9, CH | 3.67, m | 79.8, CH | 3.67, m | 78.0 |
| C5 | 74.0, CH | 3.85, m | 74.0, CH | 3.85, m | 74.0, CH | 3.85, m | 72.2 |
| C6 | 63.5, CH2 | 3.84, m | 63.2, CH2 | 3.84, m | 63.4, CH2 | 3.84, m | 61.5 |
| D1 | 102.5, CH | 5.41, d (3.5) | 102.5, CH | 5.41, d (3.5) | 102.5, CH | 5.41, d (3.5) | 100.7 |
| D2 | 74.4, CH | 3.67, m | 74.4, CH | 3.67, m | 74.4, CH | 3.66, m | 72.6 |
| D3 | 76.2, CH | 3.95, m | 76.2, CH | 3.96, m | 76.2, CH | 3.96, m | 74.4 |
| D4 | 80.7, CH | 3.67, m | 80.7, CH | 3.67, m | 80.8, CH | 3.66, m | 78.0 |
| D5 | 71.7, CH | 4.05, m | 71.8, CH | 4.05, m | 71.8, CH | 4.06, m | 72.2 |
| D6a | 66.5, CH2 | 4.43, dd (12.5, 2.0) | 66.3, CH2 | 4.44, dd (12.5, 2.0) | 66.5, CH2 | 4.50, dd (12.5, 2.0) | 61.5 |
| D6b | 4.24, brd (12.5) | 4.24, brd (12.5) | 4.26, brd (12.5) | ||||
| E1 | 103.4, CH | 5.30, d (3.5) | 103.4, CH | 5.29, d (3.5) | 103.4, CH | 5.28, d (3.5) | 101.0 |
| E2 | 74.4, CH | 3.59, m | 74.2, CH | 3.58, m | 74.2, CH | 3.59, m | 70.7 |
| E3 | 75.47, CH | 3.62, m | 75.6, CH | 3.62, m | 75.6, CH | 3.62, m | 73.8 |
| E4 | 67.7, CH | 2.48, t (9.0) | 67.9, CH | 2.46, t (9.0) | 67.9, CH | 2.47, t (9.0) | 66.0 |
| E5 | 72.4, CH | 3.72, m | 72.7, CH | 3.71, m | 72.6, CH | 3.73, m | 70.7 |
| E6 | 20.2, CH3 | 1.32, d (6.0) | 20.2, CH3 | 1.31, d (6.0) | 20.2, CH3 | 1.32, d (6.0) | 18.4 |
| F1 | 58.8, CH | 3.54, m | 58.8, CH | 3.53, m | 58.9, CH | 3.54, m | 57.1 |
| F2 | 75.52, CH | 3.66, m | 75.7, CH | 3.66, m | 75.7, CH | 3.66, m | 74.0 |
| F3 | 75.8, CH | 3.76, m | 75.8, CH | 3.77, m | 75.8, CH | 3.76, m | 73.8 |
| F4 | 73.5, CH | 4.05, d (6.4) | 73.7, CH | 4.05, d (6.4) | 73.9, CH | 4.04, d (6.4) | 72.2 |
| F5 | 142.3, C | 141.9, C | 141.9, C | 140.1 | |||
| F6a | 64.4, CH2 | 4.23, brd (13.6) | 64.4, CH2 | 4.24, brd (13.6) | 64.4, CH2 | 4.23, brd (13.6) | 62.6 |
| F6b | 4.12, brd (13.6) | 4.12, brd (13.6) | 4.12, brd (13.6) | ||||
| F7 | 126.1, CH | 5.90, dd (5.0, 1.0) | 126.6, CH | 5.91, dd (5.0, 1.0) | 126.5, CH | 5.90, dd (5.0, 1.0) | 124.8 |
| D6-O-acetyl | 177.0, C | - | - | - | - | - | |
| 23.1, CH3 | 2.16, s | - | - | - | - | - | |
| D6-O-propionyl | - | - | 180.3, C | - | - | - | |
| - | - | 30.0, CH2 | 2.48, q (7.5) | - | - | - | |
| - | - | 11.1, CH3 | 1.13, t (7.5) | - | - | - | |
| D6-O-2-hydroxy-butyryl | - | - | - | - | 176.6, C | - | |
| - | - | - | - | 45.9, CH2 | 2.67, dd (15.0, 4.5) 2.59, dd (15.0, 8.0) | - | |
| - | - | - | - | 67.3, CH2 | 4.28, m | - | |
| - | - | - | - | 24.8, CH3 | 1.26, d (6.0) | - | |
| Test Samples | IC50 (μM) | Test samples | IC50 (μM) |
|---|---|---|---|
| 1 | 0.029 ± 0.001 | 3 | 0.693 ± 0.097 |
| 2 | 0.049 ± 0.016 | 4 | 0.625 ± 0.024 |
| 6 | 0.029 ± 0.004 | 5 | 0.495 ± 0.063 |
| acarbose a | 15.7 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.-L.; E, H.-C.; Xie, D.-A.; Cheng, W.-B.; Tao, W.-Q.; Wang, Y. Acylated Aminooligosaccharides with Inhibitory Effects against α-Amylase from Streptomyces sp. HO1518. Mar. Drugs 2018, 16, 403. https://doi.org/10.3390/md16110403
Liu H-L, E H-C, Xie D-A, Cheng W-B, Tao W-Q, Wang Y. Acylated Aminooligosaccharides with Inhibitory Effects against α-Amylase from Streptomyces sp. HO1518. Marine Drugs. 2018; 16(11):403. https://doi.org/10.3390/md16110403
Chicago/Turabian StyleLiu, Hai-Li, Heng-Chao E, Ding-An Xie, Wen-Bo Cheng, Wan-Qi Tao, and Yong Wang. 2018. "Acylated Aminooligosaccharides with Inhibitory Effects against α-Amylase from Streptomyces sp. HO1518" Marine Drugs 16, no. 11: 403. https://doi.org/10.3390/md16110403