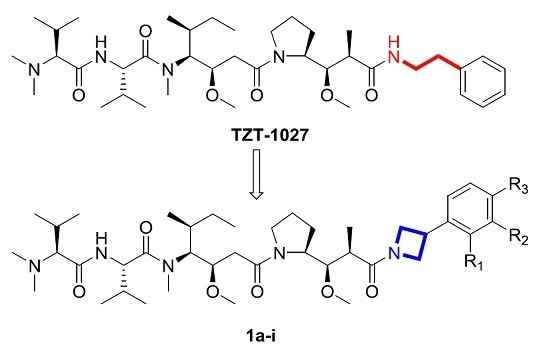
3.1. Chemistry
3.1.1. General
All starting materials, reagents, and solvents were commercially available. All reactions were monitored by thin-layer chromatography on silica gel plates (GF-254) and visualized with UV light. All the melting points were determined on a micromelting-point apparatus and thermometer was uncorrected. 1H-NMR spectra and 13C-NMR were recorded in acetone-d6 or CDCl3 on a 400 or 600 Bruker NMR spectrometer with tetramethylsilane (TMS) as an internal reference. All chemical shifts are reported in parts per million (ppm). High-resolution exact mass measurements were performed using electrospray ionization (positive mode) on a quadrupole time-of-flight (QTOF) mass spectrometer (Maxis Q-TOF, Bruker Inc., Billerica, MA, USA).
3.1.2. General Synthesis for 3-Aryl-Azetidines 5a–i
To a solution of sulfonyl chloride (1.0 equiv) in THF (0.2 M) at 0 °C was added hydrazine hydrate (2.5 equiv) dropwise. The reaction mixture was stirred at 0 °C until complete conversion was observed by thin-layer chromatography. The mixture was diluted with EtOAc, washed with brine, dried over Na2SO4 and solvents removed in vacuo to give sulfonylhydrazides. To a solution of sulfonylhydrazones (1.0 equiv) in MeOH (0.5 M) was added ketone (1.0 equiv). The reaction mixture was stirred at room temperature until complete conversion was observed by TLC. Solvents were removed in vacuo to give sulfonylhydrazones. Sulfonylhydrazone (0.5 mmol, 1.0 equiv), boronic acid (0.75 mmol, 1.5 equiv), and cesium carbonate (0.75 mmol, 1.5 equiv) were placed in an oven-dried tube in vacuo for 30 min. The tube was backfilled with argon followed by the addition of dry degassed 1,4-dioxane (2 mL, 0.25 M). This tube was sealed and heated to 110 °C for 18 h before being cooled to room temperature, quenched with NaHCO3 (2 mL of a saturated aqueous solution), and extracted with CH2Cl2 (3 × 5 mL). The organic phase was dried over MgSO4, and solvents were removed in vacuo to give a residue, which was purified by flash column chromatography (10%−30% EtOAc/hexane) to give the title compounds.
tert-Butyl 3-phenylazetidine-1-carboxylate (5a). Colorless oil; yield 57%; 1H-NMR (400 MHz, CDCl3) δ 7.39–7.29 (m, 4H), 7.27–7.23 (m, 1H), 4.33 (t, J = 8.6 Hz, 2H), 3.98 (t, J = 8.6 Hz, 2H), 3.73 (tt, J = 8.6, 6.0 Hz, 1H), 1.47 (s, 3H); 13C-NMR (150 MHz, CDCl3) δ 156.5, 142.3, 128.8, 127.0, 126.8, 79.6, 33.6, 28.5; HRMS (ESI) calcd for C14H19NO2Na: 256.1308, found: 256.1307.
tert-Butyl 3-(2-fluorophenyl)azetidine-1-carboxylate (5b). Colorless oil, yield 69%; 1H-NMR (400 MHz, CDCl3) δ 7.31 (d, J = 7.8 Hz, 1H), 7.08 (d, J = 7.7 Hz, 1H), 7.02 (d, J = 9.9 Hz, 1H), 6.96 (t, J = 8.3 Hz, 1H), 4.33 (t, J = 8.7 Hz, 2H), 3.99–3.92 (m, 2H), 3.72–3.70 (m, 1H), 1.47 (s, 9H); 13C-NMR (150 MHz, CDCl3) δ 161.7, 160.1, 156.5, 128.8, 128.7, 128.6, 128.6, 128.0, 127.9, 124.4, 115.6, 115.4, 79.6, 29.8, 28.5, 27.6, 27.5; HRMS (ESI) calcd for C14H18NO2FNa: 274.1214, found: 256.1215.
tert-Butyl 3-(3-fluorophenyl)azetidine-1-carboxylate (5c). Colorless oil, yield 62%; 1H-NMR (400 MHz, CDCl3) δ 7.31 (td, J = 7.9, 6.1 Hz, 1H), 7.08 (d, J = 7.7 Hz, 1H), 7.03 (dt, J = 10.0, 2.1 Hz, 1H), 6.96 (td, J = 8.6, 2.9 Hz, 1H), 4.33 (t, J = 8.7 Hz, 2H), 3.96 (dd, J = 8.6, 5.9 Hz, 2H), 3.77–3.68 (m, 1H), 1.42 (s, 9H); 13C-NMR (150 MHz, CDCl3) δ 163.2, 161.6, 155.7, 144.1, 144.1, 129.7, 129.6, 129.5, 121.7, 121.7, 113.3, 113.2, 113.1, 79.1, 76.5, 76.3, 76.1, 32.6, 27.7, 27.6; HRMS (ESI) calcd for C14H18NO2FNa: 274.1214, found: 256.1215.
tert-Butyl 3-(2-isopropylphenyl)azetidine-1-carboxylate (5d). Colorless oil, yield 57%; 1H-NMR (400 MHz, CDCl3) δ 7.45–7.39 (m, 1H), 7.31–7.20 (m, 3H), 4.31 (t, J = 8.0 Hz, 2H), 4.13–4.05 (m, 1H), 4.03 (m, 2H), 1.46 (s, 10H), 1.21 (s, 3H), 1.19 (s, 3H); 13C-NMR (150 MHz, CDCl3) δ 156.6, 146.6, 138.3, 127.2, 126.3, 125.7, 125.4, 79.6, 29.8, 29.1, 28.5, 23.9; HRMS (ESI) calcd for C17H25NO2Na: 298.1778, found: 258.1777.
tert-Butyl 3-(4-chlorophenyl)azetidine-1-carboxylate (5e). Colorless oil, yield 50%; 1H-NMR (400 MHz, CDCl3) δ 7.30 (d, J = 8.1 Hz, 2H), 7.22 (d, J = 8.1 Hz, 2H), 4.31 (t, J = 8.7 Hz, 2H), 3.91 (dd, J = 8.6, 5.8 Hz, 2H), 3.72–3.63 (m, 1H), 1.46 (s, 9H); 13C-NMR (150 MHz, CDCl3) δ 161.0, 159.3, 155.7, 127.9, 127.8, 127.2, 127.2, 123.7, 123.6, 114.8, 114.7, 78.9, 27.7; HRMS (ESI) calcd for C14H18ClNO2Na: 290.0918, found: 290.0916.
tert-Butyl 3-(4-(tert-butyl)phenyl)azetidine-1-carboxylate (5f). Colorless oil, yield 53%; 1H-NMR (400 MHz,CDCl3) δ 7.38 (d, J = 8.3 Hz, 2H), 7.25 (d, J = 8.1 Hz, 2H), 4.31 (t, J = 8.6 Hz, 2H), 4.02–3.93 (m, 2H), 3.71–3.69 (m, 1H), 1.47 (s, 9H), 1.32 (s, 9H); 13C-NMR (150 MHz, CDCl3) δ 155.8, 149.2, 138.5, 125.8, 124.9, 78.8, 33.8, 32.4, 30.7, 27.8; HRMS (ESI) calcd for C18H27NO2Na: 312.1934, found: 312.1936.
tert-Butyl 3-([1,1′-biphenyl]-4-yl)azetidine-1-carboxylate (5g). Colorless oil, yield 66%; 1H-NMR (400 MHz, CDCl3) δ 7.58 (d, J = 7.8 Hz, 4H), 7.44 (t, J = 7.5 Hz, 2H), 7.39–7.32 (m, 3H), 4.35 (t, J = 8.6 Hz, 2H), 4.08–3.97 (m, 2H), 3.78–3.75 (m, 1H), 1.48 (s, 9H); 13C-NMR (150 MHz, CDCl3) δ 155.8, 140.6, 140.0, 139.3, 128.1, 128.1, 126.8, 126.6, 126.5, 126.4, 78.9, 27.8; HRMS (ESI) calcd for C20H23NO2Na: 332.1621, found: 332.1623.
tert-Butyl 3-(3-chloro-4-fluorophenyl)azetidine-1-carboxylate (5h). Colorless oil, yield 55%; 1H-NMR (400 MHz, CDCl3) δ 7.39–7.34 (m, 1H), 7.21–7.15 (m, 1H), 7.12 (t, J = 7.8 Hz, 1H), 4.33 (t, J = 8.7 Hz, 2H), 3.95–3.87 (m, 2H), 3.73–3.63 (m, 1H), 1.47 (s, 9H); 13C-NMR (150 MHz, CDCl3) δ 156.3, 139.3, 129.0, 126.5, 126.45, 121.2, 117.2, 116.8, 116.7, 114.7, 79.8, 56.5, 32.7, 28.4; HRMS (ESI) calcd for C14H17ClFNO2Na: 308.0830, found: 308.0829.
tert-Butyl 3-(4-chloro-2-methoxyphenyl)azetidine-1-carboxylate (5i). Colorless oil, yield 62%; 1H-NMR (400 MHz, CDCl3) δ 6.94 (dd, J = 8.1, 1.8 Hz, 1H), 6.83 (d, J = 1.7 Hz, 1H), 4.25 (t, J = 8.4 Hz, 2H), 4.04–3.86 (m, 3H), 3.80 (s, 3H), 1.45 (s, 9H); 13C-NMR (150 MHz, CDCl3) δ 157.2, 155.9, 132.6, 127.6, 127.1, 119.8, 110.4, 78.7, 54.9, 27.9, 27.8; HRMS (ESI) calcd for C15H20ClNO3Na: 320.1024, found: 320.1024.
3.1.3. General Synthesis for 7a–i
Compounds 5a–i (1 equiv.) were dissolved in 2 mL CH2Cl2/TFA (1:1, v/v) at 0 °C, and the mixture was stirred for 1 h at room temperature. The reaction was then concentrated in vacuum, followed by azeotroping with dichloromethane three times to obtain the trifluoroacetate salts. To a stirring solution of the Dap in dry dichloromethane at 0 °C were sequentially added HATU (1.5 equiv.). After 10 min, the previously prepared trifluoroacetate salts dissolved in dichloromethane was added to reaction mixture followed by the addition of DIPEA (3 equiv.). After stirring for 12 h at room temperature, the reaction mixture was diluted with EtOAc/CH2Cl2, washed with 1M HCl, saturated NaHCO3 solution, water and brine, dried, filtered and concentrated in vacuo. Purification by silica gel column chromatography (EtOAc/Petroleum ether, 1/1) afforded compounds 7a–i.
tert-Butyl (S)-2-((1R,2R)-1-methoxy-2-methyl-3-oxo-3-(3-phenylazetidin-1-yl)propyl)pyrrolidine-1-carboxylate (7a). Colorless oil, yield 57%; 1H-NMR (400 MHz, CDCl3) δ 7.39–7.34 (m, 2H), 7.30–7.29 (m, 3H), 4.58–4.51 (m, 1H), 4.45–4.34 (m, 1H), 4.20–4.01 (m, 2H), 3.91–3.87 (m, 1H), 3.78–3.76 (m, 2H), 3.46 (s, 3H), 3.29–3.23 (m, 1H), 2.41–2.37 (m, 1H), 2.01–1.93 (m, 2H), 1.85–1.72 (m, 1H), 4.52 (d, J = 10.6 Hz, 3H), 1.25 (s, 9H); 13C-NMR (150 MHz, CDCl3) δ 148.9, 139.4, 135.0, 128.5, 128.1, 127.7, 124.8, 124.5, 120.0, 53.5, 50.9, 41.9, 41.5, 28.1, 25.8, 19.5, 18.7, 12.1, 10.8. HRMS (ESI) calcd for C23H35N2O4: 403.2591, found: 403.2597; -35.000 (CHCl3, c = 0.50).
tert-Butyl (S)-2-((1R,2R)-3-(3-(2-fluorophenyl)azetidin-1-yl)-1-methoxy-2-methyl-3-oxopropyl)pyrrolidine-1-carboxylate (7b). Colorless oil, yield 60%; 1H-NMR (400 MHz, CDCl3) δ 7.27–7.10 (m, 1H), 6.96–6.94 (m, 1H), 6.83 (d, J = 1.7 Hz, 1H), 4.50–4.43 (m, 1H), 4.35–4.31 (m, 1H), 4.27–4.24 (m, 1H), 4.19–4.08 (m, 2H), 3.97–3.82 (m, 3H), 3.81 (s, 3H), 3.78–3.74 (m, 1H), 3.26–3.22 (m,1H), 1.95 (s, 3H), 1.92–1.68 (m, 4H), 1.52 (s, 3H), 1.44 (d, J = 7.8 Hz, 3H); 13C-NMR (150 MHz, CDCl3) δ 174.4, 154.6, 129.0, 128.1, 128.0, 124.5, 115.8, 84.2, 84.1, 82.1, 79.9, 79.1, 61.2, 60.8, 59.5, 59.3, 58.9, 56.4, 53.5, 53.3, 47.0, 46.7, 39.2, 38.4, 28.7, 28.6, 27.8, 27.4, 26.2, 26.1, 25.7, 24.7, 24.6, 24.3, 14.7, 14.6, 13.8; HRMS (ESI) calcd for C23H33N2O4FNa: 443.2317, found: 443.2318; -42.000 (CHCl3, c = 0.50).
tert-Butyl (S)-2-((1R,2R)-3-(3-(3-fluorophenyl)azetidin-1-yl)-1-methoxy-2-methyl-3-oxopropyl)pyrrolidine-1-carboxylate (7c). Colorless oil, yield 55%; 1H-NMR (400 MHz, CDCl3) 7.31–7.27 (m, 1H), 7.07 (d, J = 7.4 Hz, 1H), 7.02–6.97 (m, 2H), 4.64–4.49 (m, 2H), 4.40–4.35 (m, 1H), 4.26–4.03 (m, 1H), 4.02–3.96 (m, 1H), 3.94–3.84 (m, 1H), 3.83–3.74 (m, 1H), 3.64–3.50 (m, 1H), 3.45 (s, 3H), 3.30–3.21 (m, 2H), 1.99–1.91 (m, 3H), 1.89–1.72 (m, 2H), [1.52 (s), 1.47 (s), 1.44 (s), total 9H], 1.24 (d, J = 6.9 Hz, 3H); 13C-NMR (150 MHz, CDCl3) δ 163.9, 163.9, 162.3, 162.2, 154.5, 130.4, 122.3, 114.1, 113.6, 113.5, 84.3, 84.1, 82.6, 82.3, 79.8, 79.1, 61.0, 60.7, 60.4, 58.7, 57.4, 57.4, 54.8, 54.6, 46.9, 46.8, 38.5, 32.8, 28.6, 26.2, 24.1, 14.5, 14.2; HRMS (ESI) calcd for C23H33N2O4FNa: 443.2317, found: 443.2321; -66.200 (CHCl3, c = 0.50).
tert-Butyl (S)-2-((1R,2R)-3-(3-(2-isopropylphenyl)azetidin-1-yl)-1-methoxy-2-methyl-3-oxopropyl)pyrrolidine-1-carboxylate (7d). Colorless oil, yield 65%; 1H-NMR (400 MHz, CDCl3) 7.37–7.35 (m, 1H), 7.28–7.24 (m, 3H), 4.56–4.51 (m, 1H), 4.43–4.34 (m, 1H), 4.22–4.07 (m, 3H), 3.88–3.77 (m, 3H), 3.57–3.55 (m, 1H), [3.45 (s) and 3.44 (s), total 3H], 3.26–3.23 (m, 1H), 3.01–2.93 (m, 1H), 2.50–2.39 (m, 1H), 1.95–1.72 (m, 6H), [1.51 (s), 1.47 (s) and 1.43 (s), total 9H], 1.25–1.19 (m, 9H); 13C-NMR (150 MHz, CDCl3) δ 173.6, 173.3, 153.9, 145.9, 137.2, 126.8, 125.6, 124.8, 124.6, 124.5, 83.6, 83.4, 81.9, 81.8, 79.2, 78.4, 60.5, 58.2, 58.1, 56.3, 56.2, 53.6, 53.4, 46.3, 46.0, 38.5, 37.9, 29.0, 28.5, 28.4, 28.0, 27.9, 25.5, 23.5, 23.2, 23.1, 13.9; HRMS (ESI) calcd for C26H41N2O4: 445.3061, found: 445.3065; -46.200 (CHCl3, c = 0.50).
tert-Butyl (S)-2-((1R,2R)-3-(3-(4-chlorophenyl)azetidin-1-yl)-1-methoxy-2-methyl-3-oxopropyl)pyrrolidine-1-carboxylate (7e). Colorless oil, yield 45%; 1H-NMR (400 MHz, CDCl3) 7.33–7.32 (m, 2H), 7.24–7.22 (m, 2H), 4.58–4.36 (m, 2H), 4.17–3.75 (m, 5H), 3.60–3.55 (m, 1H), [3.45 (s) and 3.44 (s), total 3H], 3.30–3.24 (m, 1H), 2.47–2.40 (m, 1H), 1.95–1.74 (m, 6H), [1.52 (s), 1.47 (s) and 1.44 (s), total 9H], 1.23 (d, J = 7.4 Hz, 3H); 13C-NMR (150 MHz, CDCl3) δ 173.9, 173.6, 173.4, 153.8, 139.6, 139.4, 132.4, 128.3, 127.3, 83.6, 83.4, 81.9, 81.7, 79.2, 78.4, 60.4, 60.1, 58.1, 56.9, 54.2, 54.1, 46.3, 46.0, 37.9, 37.8, 31.9, 29.0, 28.0, 25.6, 23.5; HRMS (ESI) calcd for C23H33ClN2O4Na: 459.2021, found: 459.2024; -46.600 (CHCl3, c = 0.50).
tert-Butyl (S)-2-((1R,2R)-3-(3-(4-(tert-butyl)phenyl)azetidin-1-yl)-1-methoxy-2-methyl-3-oxopropyl)pyrrolidine-1-carboxylate (7f). Colorless oil, yield 73%; 1H-NMR (400 MHz, CDCl3) δ 7.39 (d, J = 6.8 Hz, 2H), 7.24 (d, J = 8.1 Hz, 2H), 4.56–4.51 (m, 1H), 4.43–4.32 (m, 1H), 4.17–4.01 (m, 3H), 3.89–3.74 (m, 3H), 3.60–3.55 (m, 1H), [3.46 (s) and 3.45 (s), total 3H], 3.30–3.25 (m, 1H), 2.47–2.39 (m, 2H), 1.98–1.74 (m, 6H), [1.52 (s), 1.47 (s) and 1.45 (s), total 9H], [1.32 (s) and 1.31 (s), total 9H], 1.24 (d, J = 7.4 Hz, 3H); 13C-NMR (150 MHz, CDCl3) δ 173.9, 173.7, 173.6, 173.3, 153.8, 149.6, 149.5, 138.1, 137.9, 125.7, 125.1, 83.6, 83.4, 81.9, 81.8, 79.2, 78.4, 60.50, 58.1, 57.1, 54.4, 54.2, 46.0, 37.8, 33.8, 32.1, 31.9, 30.6, 28.0, 27.9, 25.6, 25.5, 23.6, 13.9; HRMS (ESI) calcd for C27H43N2O4: 4059.3217, found: 459.3220; -73.200 (CHCl3, c = 1).
tert-Butyl (S)-2-((1R,2R)-3-(3-([1,1′-biphenyl]-4-yl)azetidin-1-yl)-1-methoxy-2-methyl-3-oxopropyl)pyrrolidine-1-carboxylate (7g). Colorless oil, yield 45%; 1H-NMR (400 MHz, CDCl3) δ 7.59 (d, J = 7.1 Hz, 4H), 7.45 (t, J = 7.6 Hz, 3H), 7.46–7.33 (m, 3H), 4.60–4.53 (m, 1H), 4.47–4.36 (m, 1H), 4.25–4.04 (m, 3H), 3.89–3.79 (m, 3H), 3.60–3.55 (m, 1H), 3.46 (s, 3H), 3.28–3.25 (m, 1H), 2.53–2.41 (m, 1H), 1.97–1.75 (m, 5H), [1.52 (s), 1.48 (s) and 1.44 (s), total 9H], 1.25 (d, J = 7.4 Hz, 3H); 13C-NMR (150 MHz, CDCl3) δ 173.6, 173.4, 153.8, 139.9, 139.6, 128.2, 126.9, 126.7, 126.3, 83.6, 83.4, 81.9, 81.8, 79.2, 78.5, 60.5, 58.1, 57.1, 57.0, 54.3, 46.0, 37.8, 32.2, 32.1, 28.0, 27.9, 25.6, 23.5, 14.0. HRMS (ESI) calcd for C29H38N2O4: 479.2904, found: 4479.2910; -36.500 (CHCl3, c = 0.50).
tert-Butyl (S)-2-((1R,2R)-3-(3-(3-chloro-4-fluorophenyl)azetidin-1-yl)-1-methoxy-2-methyl-3-oxopropyl)pyrrolidine-1-carboxylate (7h). Colorless oil, yield 63%; 1H-NMR (400 MHz, acetone-d6) δ 7.58–7.54 (m, 1H), 7.39–7.34 (m, 2H), 4.57–4.45 (m, 1H), 4.24–4.02 (m, 4H), 3.88–3.59 (m, 5H), 3.31 (s, 3H), 3.15–3.09 (m, 1H), 1.82–1.64 (m, 6H), 1.39 (s, 9H), 0.06 (d, J = 7.4 Hz, 3H); 13C-NMR (150 MHz, acetone-d6) δ 173.1, 172.9, 156.7, 155.1, 153.1, 140.1, 139.7, 139.7, 129.4, 128.9, 128.7, 127.4, 127.3, 127.2, 119.4, 119.3, 119.2, 116.9, 116.8, 116.7, 83.7, 83.6, 81.8, 81.7, 78.4, 78.0, 60.3, 60.1, 58.6, 58.2, 56.8, 56.4, 54.2, 46.4, 46.2, 31.3, 28.0, 25.4, 24.9, 23.9, 23.4, 14.0; HRMS (ESI) calcd for C23H32ClFN2O4Na: 477.1927, found: 477.1927; -35.000 (CHCl3, c = 0.50).
tert-Butyl (S)-2-((1R,2R)-3-(3-(4-chloro-2-methoxyphenyl)azetidin-1-yl)-1-methoxy-2-methyl-3-oxopropyl)pyrrolidine-1-carboxylate (7i). Colorless oil, yield 44%; 1H-NMR (400 MHz, CDCl3) δ 7.12–7.069 (m, 1H), 6.95–6.92 (m, 1H), 6.85–6.84 (m, 1H), 4.48–4.43 (m, 1H), 4.33–4.05 (m, 3H), 3.96–3.81 (m, 2H), 3.80 (s, 3H), 3.77–3.73 (m, 1H), 3.56–3.54 (m, 1H), [3.44 (s) and 3.43 (s), total 3H], 1.95–1.51 (m, 5H), [1.50 (s), 1.46 (s) and 1.42 (s), total 9H], [1.22 (d, J = 6.9 Hz) and 1.18 (d, J = 6.9 Hz), total 3H]; 13C-NMR (150 MHz, CDCl3) δ 173.6, 157.3, 153.8, 127.3, 127.2, 119.9, 110.6, 83.4, 83.3, 81.8, 79.2, 78.4, 60.5, 58.2, 58.1, 55.2, 54.9, 54.9, 52.0, 51.9, 46.3, 46.0, 37.9, 29.0, 28.0, 27.9, 25.5, 23.5, 14.0, 13.8; HRMS (ESI) calcd for C24H36N2O5: 467.2307, found: 467.2311; -32.300 (CHCl3, c = 0.50).
3.1.4. General Synthesis for 1a–i
Commercially available tripeptide (1 equiv.) and 7a–i (1 equiv.) was dissolved in CH2Cl2/TFA (1:1, v/v). After stirred for 2 h at room temperature, the solvent was removed in vacuum, followed by azeotroping with CH2Cl2 three times. Then the mixture was dissolved in dry CH2Cl2. DIPEA was added until reaction mixture was basic, followed by HATU (1.5 equiv.). After stirring for 12 h at room temperature, the reaction mixture was diluted with EtOAc, washed with 1M HCl, saturated NaHCO3 solution, water and brine, dried, filtered, and concentrated in vacuum. Purification by silica gel column chromatography (CH2Cl2/MeOH, 20/1) afforded the title compounds 1a–i.
(S)-2-((R)-2-(Dimethylamino)-3-methylbutanamido)-N-((3R,4S,5S)-3-methoxy-1-((S)-2-((1R,2R)-1-methoxy-2-methyl-3-oxo-3-(3-phenylazetidin-1-yl)propyl)pyrrolidin-1-yl)-5-methyl-1-oxoheptan-4-yl)-N,3-dimethylbutanamide (1a). Colorless oil, yield 45%; 1H-NMR (400 MHz, CDCl3) 7.40–7.36 (m, 2H), 7.31–7.27 (m, 3H), 6.93 (d, J = 9.1 Hz, 1H), [4.87 (t, J = 7.2 Hz) and 4.79 (t, J = 7.8 Hz), total 1H], 4.66–4.59 (m, 1H), 4.54–4.46 (m, 1H), 4.45–4.40 (m, 1H), 4.37–4.32 (m, 1H), 4.28–4.23 (m, 1H), 4.21–3.77 (m, 11H), [3.43 (s) and 3.41 (s), total 3H], [3.36 (s) and 3.35 (s), total 3H], [3.34 (s) and 3.30 (s), total 3H], 3.14 (d, J = 6.2 Hz, 1H), 3.03–3.02 (m, 2H), 2.63–0.79 (m, 36H); 13C-NMR (150 MHz, CDCl3) 174.4, 147.1, 173.8, 173.7, 173.1, 171.2, 170.2, 169.9, 161.8, 161.7, 160.1, 129.2, 129.1, 129.0, 128.8, 128.4, 128.2, 128.1, 127.9, 127.7, 124.5, 124.4, 116.0, 115.8, 115.7, 115.6, 115.5, 86.4, 82.6, 82.4, 78.3, 77.8, 76.0, 61.9, 61.8, 60.5, 60.4, 59.4, 59.2, 59.0, 58.1, 57.9, 57.8, 56.5, 56.4, 56.2, 56.0, 55.8, 53.8, 53.6, 53.4, 53.2, 47.7, 47.5, 46.6, 46.5, 42.6, 39.4, 38.8, 37.7, 37.4, 35.8, 33.2, 33.1, 32.3, 31.8, 30.9, 28.4, 27.8, 27.7, 27.3, 26.2, 26.0, 25.7, 25.0, 24.9, 24.6, 23.6, 23.5, 20.1, 19.8, 19.5, 17.9, 17.8, 15.8, 15.4, 14.8, 14.6, 13.7, 13.5, 10.8, 10.7, 10.3; HRMS (ESI) calcd for C40H68N5O6: 714.5164, found: 714.5169; -50.200 (MeOH, c = 0.50).
(S)-2-((R)-2-(Dimethylamino)-3-methylbutanamido)-N-((3R,4S,5S)-1-((S)-2-((1R,2R)-3-(3-(2-fluorophenyl)azetidin-1-yl)-1-methoxy-2-methyl-3-oxopropyl)pyrrolidin-1-yl)-3-methoxy-5-methyl-1-oxoheptan-4-yl)-N,3-dimethylbutanamide (1b). Colorless oil, yield 40%; 1H-NMR (400 MHz, acetone-d6) δ 7.45–7.38 (m, 1H), 7.36–7.31 (m 1H), 7.23–7.19 (m, 1H), 7.16–7.11 (m, 1H), 4.80–4.63 (m, 3H), 4.45–4.33 (m, 2H), 4.15–3.93 (m, 4H), 3.68–3.55 (m, 2H), [3.42 (s) and 3.33 (s), total 3H], [3.30 (s) and 3.13 (s), total 3H], 2.75–2.05 (m, 10H), 1.20–0.79 (m, 24H); 13C-NMR (150 MHz, acetone-d6) δ 174.2, 174.2, 172.4, 170.1, 161.2, 159.6, 128.5, 128.4, 128.4, 128.0, 127.9, 127.5, 127.4, 127.3, 125.5, 124.0, 114.8, 114.7, 114.6, 81.4, 81.3, 78.7, 73.9, 60.2, 59.0, 58.9, 56.2, 55.2, 53.8, 52.9, 47.1, 47.0, 41.1, 38.2, 37.2, 36.0, 31.7 30.5, 29.6, 29.1, 27.1, 27.0, 26.6, 25.0, 24.2, 23.4, 18.5, 18.1, 17.6, 17.1, 16.9, 14.8, 13.3; HRMS (ESI) calcd for C40H67FN5O6: 732.5070, found: 732.5088; -35.800 (CHCl3, c = 0.50).
(S)-2-((R)-2-(Dimethylamino)-3-methylbutanamido)-N-((3R,4S,5S)-1-((S)-2-((1R,2R)-3-(3-(3-fluorophenyl)azetidin-1-yl)-1-methoxy-2-methyl-3-oxopropyl)pyrrolidin-1-yl)-3-methoxy-5-methyl-1-oxoheptan-4-yl)-N,3-dimethylbutanamide (1c). Colorless oil, yield 54%; 1H-NMR (400 MHz, CDCl3) δ 7.29–7.23 (m, 2H), 7.19–7.11 (m, 1H), 7.06–6.97 (m, 1H), 4.85–4.74 (m, 1H), 4.60–4.54 (m, 1H), 4.52–4.73 (m, 1H), 4.41–4.34 (m, 1H), 4.23–4.17 (m, 2H), 4.12–3.85 (m, 5H), 3.48–3.45 (m, 2H), [3.41 (s) and 3.40 (s), total 3H], [3.31 (s) and 3.29 (s), total 3H], 3.05 (s, 3H), 2.64–2.38 (m, 4H), 2.37–2.27 (m, 6H), 2.18–1.77 (m, 10H), 1.37–0.76 (m, 21H); 13C-NMR (150 MHz, CDCl3) δ 174.1, 174.0, 172.4, 170.6, 169.9, 169.6, 161.2, 159.6, 159.4, 128.7, 128.3, 128.3, 127.8, 127.2, 127.1, 126.9, 124.1, 123.8, 115.0, 114.9, 114.8, 114.7, 81.3, 79.0, 75.4, 68.9, 60.6, 59.0, 58.9, 58.4, 57.5, 56.8, 56.8, 56.0, 55.6, 53.1, 52.7, 52.6, 47.2, 42.0, 42.0, 38.5, 38.3, 36.2, 32.0, 31.1, 30.8, 30.2, 29.0, 28.6, 27.3, 26.9, 25.0, 24.3, 24.2, 23.8, 23.7, 19.4, 19.3, 19.1, 18.7, 17.6, 17.0, 15.1, 13.7, 13.6; HRMS (ESI) calcd for C40H67FN5O6: 732.5070, found: 732.5078; -35.200 (MeOH, c = 0.50).
(S)-2-((R)-2-(Dimethylamino)-3-methylbutanamido)-N-((3R,4S,5S)-1-((S)-2-((1R,2R)-3-(3-(2-isopropylphenyl)azetidin-1-yl)-1-methoxy-2-methyl-3-oxopropyl)pyrrolidin-1-yl)-3-methoxy-5-methyl-1-oxoheptan-4-yl)-N,3-dimethylbutanamide (1d). Colorless oil, yield 48%; 1H-NMR (400 MHz, CDCl3) δ 7.45–7.40 (m, 1H), 7.33–7.28 (m, 2H), 7.26–7.17 (m, 1H), 5.11–4.69 (m, 3H), 4.35–4.22 (m, 4H), 4.06–4.02 (m, 3H), 3.57–3.55 (m, 2H), [3.40 (s) and 3.38 (s), total 3H), 3.39–3.01 (m, 10H), 2.66–2.45 (m, 8H), 2.20–1.95 (m, 6H), 1.88–1.71 (m, 4H), 1.41–1.35 (m, 2H), 1.21–1.13 (m, 8H), 1.02–0.76 (m, 19H); 13C-NMR (150 MHz, CDCl3) δ 173.9, 173.6, 173.1, 173.0, 172.4, 169.7, 169.6, 169.0, 167.6, 167.5, 159.9, 159.7, 148.0, 145.9, 145.8, 139.2, 137.3, 134.3, 127.3, 126.5, 126.4, 126.4, 125.5, 125.5, 124.8, 124.7, 124.6, 124.6, 124.5, 124.5, 118.8, 117.5, 115.5, 81.56 81.4, 72.6, 72.5, 60.3, 59.8, 59.0, 58.9, 58.3, 56.7, 56.6, 56.3, 56.3, 55.9, 54.0, 53.7, 53.6, 53.4, 46.9, 45.4, 45.3, 40.4, 40.4, 38.3, 38.2, 38.0, 31.8, 29.8, 29.7, 29.0, 28.9, 28.8, 26.8, 26.8, 25.4, 25.3, 25.0, 24.1, 24.0, 23.8, 23.7, 22.8, 22.6, 18.5, 18.4, 18.1, 17.6, 17.5, 17.3, 17.3, 14.8, 13.5, 13.0, 12.7, 9.4; HRMS (ESI) calcd for C43H74N5O6: 778.5453, found: 778.5453; -40.200 (CHCl3, c = 1).
(S)-N-((3R,4S,5S)-1-((S)-2-((1R,2R)-3-(3-(4-Chlorophenyl)azetidin-1-yl)-1-methoxy-2-methyl-3-oxopropyl)pyrrolidin-1-yl)-3-methoxy-5-methyl-1-oxoheptan-4-yl)-2-((R)-2-(dimethylamino)-3-methylbutanamido)-N,3-dimethylbutanamide (1e). Colorless oil, yield 66%; 1H-NMR (400 MHz, CDCl3) δ 7.44–7.37 (m, 4H), 4.83–4.67 (m, 3H), 4.40–4.13 (m, 4H), 4.02–3.83 (m, 4H), 3.60–3.55 (m, 3H), [3.40 (s) and 3.37 (s), total 3H], [3.31 (s) and 3.26 (s), total 3H], 3.22–3.09 (m, 4H), 2.68–2.39 (m, 5H), 2.28 (s, 3H), 2.27 (s, 3H), 2.14–1.80 (m, 10H), 1.20–1.13 (m, 4H), 1.02–0.79 (m, 21H); 13C-NMR (150 MHz, CDCl3) δ 173.2, 172.9, 172.7, 169.7, 169.6, 168.8, 168.7, 141.1, 140.8, 140.6, 140.5, 131.5, 131.4, 128.1, 128.0, 127.9, 127.8, 85.7, 85.5, 81.8, 77.8, 77.6, 77.2, 74.3, 74.2, 60.3, 59.1, 58.6, 58.5, 58.2, 58.1, 56.6, 56.5, 54.2, 54.1, 54.0, 53.9, 53.2, 53.1, 46.5, 46.3, 45.6, 41.0, 38.5, 38.0, 37.9, 36.8, 36.5, 35.2, 31.9, 31.7, 29.9, 28.7, 28.6, 28.5, 28.3, 28.2, 28.1, 27.9, 26.6, 25.5, 25.4, 25.0, 24.9, 24.4, 24.0, 23.7, 22.8, 18.7, 18.5, 18.2, 17.8 17.4, 17.3, 14.8, 14.5, 13.6, 13.5, 12.4, 11.9, 9.5, 9.2; HRMS (ESI) calcd for C40H67ClN5O6: 748.4774, found: 748.4778; -34.500 (CHCl3, c = 0.50).
(S)-N-((3R,4S,5S)-1-((S)-2-((1R,2R)-3-(3-(4-(tert-Butyl)phenyl)azetidin-1-yl)-1-methoxy-2-methyl-3-oxopropyl)pyrrolidin-1-yl)-3-methoxy-5-methyl-1-oxoheptan-4-yl)-2-((R)-2-(dimethylamino)-3-methylbutanamido)-N,3-dimethylbutanamide (1f). Colorless oil, yield 35%; 1H-NMR (400 MHz, CDCl3) δ 7.44–7.35 (m, 2H), 7.26–7.15 (m, 2H), 4.86–4.69 (m, 3H), 4.64–4.51 (m, 2H), 4.22–4.01 (m, 2H), 4.01–3.76 (m, 4H), 3.75–3.66 (m, 4H), 3.55–3.47 (m, 5H), [3.42 (s) and 3.39 (s), total 3H], [3.29 (s) and 3.28 (s), total 3H], 3.24–3.11 (m, 3H), 3.03–2.62 (m, 1H), 2.59–2.27 (m, 4H), 2.21–1.69 (m, 3H), 1.47–1.15 (m, 10H), 1.05–0.71 (m, 18H); 13C-NMR (150 MHz, CDCl3) δ 174.0, 173.9, 172.5, 170.1, 170.0, 149.8, 149.7, 137.5, 137.2, 125.6, 125.1, 81.2, 81.0, 78.8, 60.7, 59.2, 59.0, 57.5, 57.2, 56.8, 54.8, 54.6, 47.6, 47.4, 42.8, 41.9, 41.8, 38.5, 38.4, 33.8, 31.9, 31.5, 31.3, 30.6, 30.2, 28.6, 26.9, 25.1, 24.3, 24.2, 23.8, 19.2, 19.0, 18.4, 18.4, 17.8, 17.0, 16.6, 15.1, 14.0, 13.8, 12.0, 10.0; HRMS (ESI) calcd for C44H76N5O6: 770.5790, found: 770.5809; -36.000 (CHCl3, c = 0.50).
(S)-N-((3R,4S,5S)-1-((S)-2-((1R,2R)-3-(3-([1,1′-Biphenyl]-4-yl)azetidin-1-yl)-1-methoxy-2-methyl-3-oxopropyl)pyrrolidin-1-yl)-3-methoxy-5-methyl-1-oxoheptan-4-yl)-2-((R)-2-(dimethylamino)-3-methylbutanamido)-N,3-dimethylbutanamide (1g). Colorless oil, yield 70%; 1H-NMR (400 MHz, CDCl3) δ 7.60 (d, J = 8.4 Hz, 4H), 7.47–7.44 (m, 2H), 7.40–7.34 (m, 3H), 4.89–4.79 (m, 3H), 4.68–4.65 (m, 1H), 4.58–4.47 (m, 1H), 4.45–4.39 (m, 1H), 4.23–3.85 (m, 10H), [3.45 (s) and 3.42 (s), total 3H], [3.37 (s) and 3.32 (s), total 3H], 3.16–3.03 (m, 4H), 2.80–2.02 (m, 10H), 1.26–0.82 (m, 23H); 13C-NMR (150 MHz, CDCl3) δ 173.8, 173.5, 169.6, 169.2, 139.8, 139.5, 128.1, 127.0, 126.9, 126.7, 126.5, 126.4, 126.3, 126.2, 81.9, 75.7,58.5, 58.4, 57.3, 57.2, 57.0, 56.9, 54.4, 53.1, 42.1, 38.2, 32.6, 32.5, 32.4, 32.2, 32.1, 31.2, 30.3, 29.0, 28.7, 27.0, 25.7, 25.1, 24.5, 24.4, 24.1, 23.0, 22.0, 19.4, 19.2, 18.9, 17.1, 15.2, 14.1, 13.4, 13.0, 10.1, 9.7; HRMS (ESI) calcd for C46H72N5O6: 790.5477, found: 790.5475; -20.900 (CHCl3, c = 1).
(S)-N-((3R,4S,5S)-1-((S)-2-((1R,2R)-3-(3-(3-Chloro-4-fluorophenyl)azetidin-1-yl)-1-methoxy-2-methyl-3-oxopropyl)pyrrolidin-1-yl)-3-methoxy-5-methyl-1-oxoheptan-4-yl)-2-((R)-2-(dimethylamino)-3-methylbutanamido)-N,3-dimethylbutanamide (1h). Colorless oil, yield 44%; 1H-NMR (400 MHz, CDCl3) δ 7.37–7.35 (m, 1H), 7.16–7.09 (m, 2H), 4.90–4.77 (m, 2H), 4.64–4.60 (m, 1H), 4.52–4.40 (m, 2H), 4.18–4.00 (m, 4H), 3.80–3.76 (m, 3H), [3.43 (s) and 3.41 (s), total 3H], [3.38 (s) and 3.36 (s), total 3H], 3.34–3.30 (m, 4H), 3.18–3.02 (m, 4H), 2.37–2.00 (m, 7H), 1.94–1.70 (m, 10H), 1.42–0.80 (m, 13H); 13C-NMR (150 MHz, CDCl3) δ 174.1, 174.0, 172.4, 170.6, 169.6, 161.2, 161.0, 159.6, 159.4, 128.3, 127.8, 127.1, 124.1, 123.8, 115.0, 114.9, 114.8, 114.7, 81.3, 79.0, 68.9, 60.6, 59.0, 58.9, 58.4, 57.5, 56.8, 56.0, 55.6, 53.1, 52.7, 52.6, 47.2, 42.0, 42.0, 38.5, 38.3, 36.2, 32.0, 31.1, 30.8, 30.2, 29.0, 28.6, 27.3, 26.9, 25.0, 24.3, 24.2, 23.8, 23.7, 19.4, 19.3, 19.1, 18.7, 17.6, 17.0, 15.1, 13.7, 13.6, 10.0; HRMS (ESI) calcd for C40H66ClFN5O6: 766.4680, found: 766.4685; -41.400 (MeOH, c = 0.50).
(S)-N-((3R,4S,5S)-1-((S)-2-((1R,2R)-3-(3-(4-Chloro-2-methoxyphenyl)azetidin-1-yl)-1-methoxy-2-methyl-3-oxopropyl)pyrrolidin-1-yl)-3-methoxy-5-methyl-1-oxoheptan-4-yl)-2-((R)-2-(dimethylamino)-3-methylbutanamido)-N,3-dimethylbutanamide (1i). Colorless oil, yield 45%; 1H-NMR (400 MHz, CDCl3) δ 7.15–7.10 (m, 1H), 7.01–6.92 (m, 1H), 6.87–6.84 (m, 1H), 4.88–4.73 (m, 3H), 4.56–4.50 (m, 1H), 4.45–4.41 (m, 1H), 4.37–4.06 (m, 4H), 3.99–3.91 (m, 2H), [3.83 (s) and 3.80 (s), total 3H], [3.44 (s) and 3.42 (s), total 3H], 3.39–3.29 (m, 5H), 3.14–3.01 (m, 4H), 2.82–2.76 (m, 1H), 2.63–2.24 (m, 7H), 2.31 (s, 3H), 2.29 (s, 3H), 2.18–1.77 (m, 9H), 1.37–1.13 (m, 10H), 1.05–0.79 (m, 9H); 13C-NMR (150 MHz, CDCl3) δ 174.4, 173.7, 173.5, 173.2, 171.2, 170.2, 157.9, 133.9, 133.6, 127.9, 127.5, 127.0, 120.6, 111.3, 86.2, 78.5, 78.0, 61.8, 59.4, 58.1, 57.8, 55.8, 53.8, 52.9, 52.6, 47.8, 46.6, 42.7, 39.3, 38.8, 37.4, 35.8, 32.9, 31.9, 28.8, 28.5, 26.0, 25.7, 24.9, 24.4, 23.6, 19.8, 19.4, 18.0, 17.8, 15.8, 13.9, 10.9; HRMS (ESI) calcd for C41H69ClN5O7: 778.4880, found: 778.4879; -33.400 (MeOH, c = 0.50).