Synthesis, Characterization and Cytotoxicity of Novel Multifunctional Fe3O4@SiO2@GdVO4:Dy3+ Core-Shell Nanocomposite as a Drug Carrier
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Materials
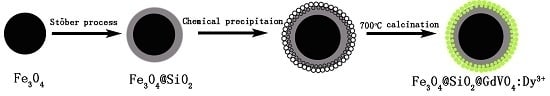
3.2. Synthesis of Fe3O4
3.3. Synthesis of Fe3O4@SiO2
3.4. Synthesis of Fe3O4@SiO2@GdVO4:Dy3+ Nanoparticles
3.5. Cytotoxicity Study of Fe3O4@SiO2@GdVO4:Dy3+ Nanoparticle
3.6. Characterization
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sun, Y.; Zhou, Y.; Li, Q.; Yang, Y. Enzyme-responsive supramolecular nanovalves crafted by mesoporous silica nanoparticles and choline-sulfonatocalix[4]arene[2]pseudorotaxanes for controlled cargo release. Chem. Commun. 2013, 49, 9033–9035. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Yang, B.; Zhang, S.X.; Yang, Y. Cucurbit[7]uril Pseudorotaxane-Based Photoresponsive Supramolecular Nanovalve. Chem. Eur. J. 2012, 18, 9212–9216. [Google Scholar] [CrossRef] [PubMed]
- Corr, S.A.; Rakovich, Y.P.; Gun’ko, Y.K. Multifunctional magnetic-fluorescent nanocomposites for biomedical applications. Nanoscale Res. Lett. 2008, 3, 87–104. [Google Scholar] [CrossRef]
- Ajithkumar, G.; Yoo, B.; Goral, D.E.; Hornsby, P.J.; Lin, A.L.; Ladiwala, U.; Dravide, V.P.; Sardara, D.K. Multimodal bioimaging using a rare earth doped Gd2O2S:Yb/Er phosphor with upconversion luminescence and magnetic resonance properties. J. Mater. Chem. B 2013, 1, 1561–1572. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.M.; Shi, J.H.; Tong, L.Z.; Ren, X.Z.; Li, Q.H.; Yang, H. YVO4:Eu3+, Dy3+@Fe3O4 co-doped nanocomposites: Preparation, luminescent, and magnetic properties. J. Nanopart. Res. 2012, 14, 1–7. [Google Scholar] [CrossRef]
- Tong, L.Z.; Liu, D.M.; Shi, J.H.; Yang, X.W.; Yang, H. Magnetic and luminescent properties of Fe3O4@Y2O3:Eu3+ nanocomposites. J. Mater. Sci. 2012, 47, 132–137. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Y.H.; Xu, Z.G.; Zhang, H.X.; Dong, P.Y.; Guo, L.N.; Li, F.H.; Xin, S.Y.; Zeng, W. Preparation and drug-delivery properties of hollow YVO4:Ln3+ and mesoporous YVO4:Ln3+@nSiO2@mSiO2 (Ln = Eu, Yb, Er, and Ho). J. Mater. Chem. B 2013, 1, 330–338. [Google Scholar] [CrossRef]
- Tong, L.Z.; Shi, J.H.; Liu, D.M.; Li, Q.H.; Ren, X.Z.; Yang, H. Luminescent and Magnetic Properties of Fe3O4@SiO2@Y2O3:Eu3+ Composites with Core–Shell Structure. J. Phys. Chem. C 2012, 116, 7153–7157. [Google Scholar] [CrossRef]
- Ren, X.Z.; Shi, J.H.; Tong, L.Z.; Li, Q.H.; Yang, H. Magnetic and luminescence properties of the porous CoFe2O4@Y2O3:Eu3+ nanocomposite with higher coercivity. J. Nanopart. Res. 2013, 15, 1–10. [Google Scholar] [CrossRef]
- Liu, Y.L.; Wang, Z.G.; Cheng, G.; Zhang, J.L.; Hong, G.Y.; Ni, J.Z. Synthesis and characterization of Fe3O4@YPO4:Eu3+ multifunctional microspheres. Mater. Lett. 2015, 152, 224–227. [Google Scholar] [CrossRef]
- Shen, J.; Sun, L.D.; Zhang, Y.W.; Yan, C.H. Superparamagnetic and upconversion emitting Fe3O4/NaYF4:Yb, Er hetero-nanoparticles via a crosslinker anchoring strategy. Chem. Commun. 2010, 46, 5731–5733. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, Y.S.; Yang, Y.; Zhang, F.; Dong, W.F.; Zhou, S.Y.; Pei, W.H.; Chen, H.D. Magnetic/upconversion luminescent mesoparticles of Fe3O4@LaF3:Yb3+, Er3+ for dual-modal bioimaging. Chem. Commun. 2012, 48, 11238–11240. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Zhou, J.; Chen, M.; Shi, M.; Feng, W.; Li, F. Core-shell Fe3O4@NaLuF4:Yb,Er/Tm nanostructure for MRI, CT and upconversion luminescence tri-modality imaging. Biomaterials 2012, 33, 4618–4627. [Google Scholar] [CrossRef] [PubMed]
- Jing, P.; Wang, Q.; Liu, B.C.; Xu, G.R.; Zhang, Y.B.; Zhang, J.; De, G. Controlled fabrication of bi-functional Fe3O4@SiO2@Gd2O3:Yb,Er nanoparticles and their magnetic, up-conversion luminescent properties. RSC Adv. 2014, 4, 44575–44582. [Google Scholar] [CrossRef]
- Gao, Y.; Zhao, Q.; Fang, Q.H.; Xu, Z.H. Facile fabrication and photoluminescence properties of rare-earth-doped Gd2O3 hollow spheres via a sacrificial template method. Dalton Trans. 2013, 42, 11082–11091. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Sun, L.D.; Yan, C.H. Luminescent rare earth nanomaterials for bioprobe applications. Dalton Trans. 2008, 14, 5687–5697. [Google Scholar] [CrossRef] [PubMed]
- Barick, K.C.; Sharma, A.; Shetake, N.G.; Ningthoujam, R.S.; Vatsa, R.K.; Babu, P.D.; Pandey, B.N.; Hassan, P.A. Covalent bridging of surface functionalized Fe3O4 and YPO4:Eu nanostructures for simultaneous imaging and therapy. Dalton Trans. 2015, 44, 14686–14696. [Google Scholar] [CrossRef] [PubMed]
- Singh, L.P.; Jadhav, N.V.; Sharma, S.; Pandey, B.N.; Srivastavaa, S.K.; Ningthoujam, R.S. Hybrid nanomaterials YVO4:Eu/Fe3O4 for optical imaging and hyperthermia in cancer cells. J. Mater. Chem. C 2015, 3, 1965–1975. [Google Scholar] [CrossRef]
- Shi, J.H.; Liu, D.M.; Tong, L.Z.; Yang, X.W.; Yang, H. Magnetic and photoluminescence properties of Fe3O4@SiO2@YP1−xVxO4:Dy3+ nanocomposites. J. Alloys Compd. 2011, 509, 10211–10216. [Google Scholar] [CrossRef]
- Parchur, A.K.; Ansari, A.A.; Singh, B.P.; Hasan, T.N.; Syed, N.A.; Raia, S.B.; Ningthoujam, R.S. Enhanced luminescence of CaMoO4:Eu by core@shell formation and its hyperthermia study after hybrid formation with Fe3O4: Cytotoxicity assessment on human liver cancer cells and mesenchymal stem cells. Integr. Biol. 2014, 6, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.H.; Moon, B.K.; Choi, B.C.; Jeong, J.H.; Kim, J.H.; Lee, H.S. The green upconversion emission mechanism investigation of GdVO4:Yb3+, Er3+ via tuning of the sensitizer concentration. Solid State Sci. 2015, 42, 1–5. [Google Scholar] [CrossRef]
- Wang, W.; Zou, M.; Chen, K.Z. Novel Fe3O4@YPO4:Re (Re = Tb, Eu) multifunctional magnetic-fluorescent hybrid spheres for biomedical applications. Chem. Commun. 2010, 46, 5100–5102. [Google Scholar] [CrossRef] [PubMed]
- Mi, C.C.; Zhang, J.P.; Gao, H.Y.; Wu, X.L.; Wang, M.; Wu, Y.F.; Di, Y.Q.; Xu, Z.R.; Mao, C.B.; Xu, S.K. Multifunctional nanocomposites of superparamagnetic (Fe3O4) and NIR-responsive rare earth-doped up-conversion fluorescent (NaYF4:Yb,Er) nanoparticles and their applications in biolabeling and fluorescent imaging of cancer cells. Nanoscale 2010, 2, 1141–1148. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.M.; Tong, L.Z.; Shi, J.H.; Yang, H. Luminescent and magnetic properties of YVO4:Ln3+@Fe3O4 (Ln3+ = Eu3+ or Dy3+) Nanocomposites. J. Alloys Compd. 2012, 512, 361–365. [Google Scholar] [CrossRef]







© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, B.; Fan, H.; Zhao, Q.; Wang, C. Synthesis, Characterization and Cytotoxicity of Novel Multifunctional Fe3O4@SiO2@GdVO4:Dy3+ Core-Shell Nanocomposite as a Drug Carrier. Materials 2016, 9, 149. https://doi.org/10.3390/ma9030149
Li B, Fan H, Zhao Q, Wang C. Synthesis, Characterization and Cytotoxicity of Novel Multifunctional Fe3O4@SiO2@GdVO4:Dy3+ Core-Shell Nanocomposite as a Drug Carrier. Materials. 2016; 9(3):149. https://doi.org/10.3390/ma9030149
Chicago/Turabian StyleLi, Bo, Huitao Fan, Qiang Zhao, and Congcong Wang. 2016. "Synthesis, Characterization and Cytotoxicity of Novel Multifunctional Fe3O4@SiO2@GdVO4:Dy3+ Core-Shell Nanocomposite as a Drug Carrier" Materials 9, no. 3: 149. https://doi.org/10.3390/ma9030149