Solution Synthesis of Cubic Spinel Mn–Ni–Cu–O Thermistor Powder
Abstract
:1. Introduction
2. Experimental Procedure
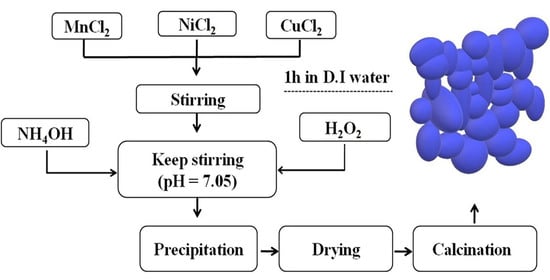
2.1. Preparation of Powder and Ceramics
2.2. Characterizations
2.2.1. Powders
2.2.2. Ceramics
3. Results and Discussion
3.1. Characterization of Powder
3.2. Crystal Structure and Microstructure of Sintered Ceramics
3.3. Electrical Properties of Sintered Ceramics
3.4. Formation Mechanism of Spinel Powder
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dinger, J.; Reimann, T.; Ovodok, E.; Töpfer, J. Cation distribution in NiMn2O4 spinel probed by high temperature thermopower measurements. J. Alloys Compd. 2021, 865, 158909. [Google Scholar] [CrossRef]
- Chen, J.; Wang, J.; Yao, J.; Chang, A.; Wang, B. Pd/Ag thin film deposited on negative temperature coefficient (NTC) ceramics by direct current magnetron sputtering. Vacuum 2019, 167, 227–233. [Google Scholar] [CrossRef]
- Feteira, A. Negative Temperature Coefficient Resistance (NTCR) Ceramic Thermistors: An Industrial Perspective. J. Am. Ceram. Soc. 2009, 92, 967–983. [Google Scholar] [CrossRef]
- Schubert, J.M.; Münch, C.; Schuurman, S.; Poulain, V.; Kita, J.; Moos, R. Characterization of nickel manganite NTC thermistor films prepared by aerosol deposition at room temperature. J. Eur. Ceram. Soc. 2018, 38, 613–619. [Google Scholar] [CrossRef]
- Liu, T.; Dai, A.; Lu, J.; Yuan, Y.; Xiao, Y.; Yu, L.; Li, M.; Gim, J.; Ma, L.; Liu, J.; et al. Correlation between manganese dissolution and dynamic phase stability in spinel-based lithium-ion battery. Nat. Commun. 2019, 10, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Metz, R. Electrical properties of N.T.C. thermistors made of manganite ceramics of general spinel structure: Mn3−x−x’ MxNx’O4 (0 ≤ x + x’ ≤ 1; M and N being Ni, Co or Cu). Aging phenomenon study. J. Mater. Sci. 2000, 35, 4705–4711. [Google Scholar] [CrossRef]
- Jeon, C.J.; Jeong, Y.H.; Yun, J.S.; Park, W.I.; Paik, J.H.; Hong, Y.W.; Kim, E.S.; Cho, J.H. Electrical properties of copper-nickel manganite thin films prepared by metal-organic decomposition. Ceram. Int. 2017, 43, 9291–9295. [Google Scholar] [CrossRef]
- Lenglet, M.; D’Huysser, A.; Kasperek, J.; Bonnelle, J.P.; Durr, J. Characterization of the oxidation states of copper and manganese in some manganites by the analysis of the XPS spectrum, X emission, and X absorption thresholds. Mater. Res. Bull. 1985, 20, 745–757. [Google Scholar] [CrossRef]
- Elbadraoui, E.; Baudour, J.L.; Bouree, F.; Gillot, B.; Fritsch, S.; Rousset, A. Cation distribution and mechanism of electrical conduction in nickel-copper manganite spinels. Solid State Ion. 1997, 93, 219–225. [Google Scholar] [CrossRef]
- de Györgyfalva, G.C.; Reaney, I.M. Decomposition of NiMn2O4 spinel: An NTC thermistor material. J. Eur. Ceram. Soc. 2001, 21, 2145–2148. [Google Scholar] [CrossRef]
- De Györgyfalva, G.D.C.C.; Reaney, I.M. Decomposition of NiMn2O4 spinels. J. Mater. Res. 2003, 18, 1301–1308. [Google Scholar] [CrossRef]
- Chang, A.; Zhang, B.; Wu, Y.; Zhao, Q.; Zhang, H.; Yao, J.; Xu, J.; Zhao, P. Spark Plasma Sintering of Negative Temperature Coefficient Thermistor Ceramics. In Sintering Techniques of Materials; InTech: Rijeka, Croatia, 2015. [Google Scholar] [CrossRef]
- de Györgyfalva, C.D.C.C.; Nolte, A.; Reaney, I. Correlation between microstructure and conductance in NTC thermistors produced from oxide powders. J. Eur. Ceram. Soc. 1999, 19, 857–860. [Google Scholar] [CrossRef]
- Yuksel, P.B.; Gokhan, H. Preparation and characterization of Ni-Co-Zn-Mn-O negative temperature coefficient thermistors with B2O3addition. J. Mater. Sci. Mater. Electron. 2019, 30, 17432–17439. [Google Scholar] [CrossRef]
- Kocjan, A.; Logar, M.; Shen, Z. The agglomeration, coalescence and sliding of nanoparticles, leading to the rapid sintering of zirconia nanoceramics. Sci. Rep. 2017, 7, 25. [Google Scholar] [CrossRef]
- Aleksić, O.; Nikolić, M.V.; Luković, M.D.; Nikolic, N.; Radojcic, B.M.; Radovanovic, M.; Djuric, Z.G.; Mitric, M.; Nikolić, P. Preparation and characterization of Cu and Zn modified nickel manganite NTC powders and thick film thermistors. Mater. Sci. Eng. B 2013, 178, 202–210. [Google Scholar] [CrossRef]
- Fang, D.-L.; Wang, Z.-B.; Yang, P.-H.; Liu, W.; Chen, C.-S.; Winnubst, A.J.A. Preparation of Ultra-Fine Nickel Manganite Powders and Ceramics by a Solid-State Coordination Reaction. J. Am. Ceram. Soc. 2006, 89, 230–235. [Google Scholar] [CrossRef]
- Arinicheva, Y.; Clavier, N.; Neumeier, S.; Podor, R.; Bukaemskiy, A.; Klinkenberg, M.; Roth, G.; Dacheux, N.; Bosbach, D. Effect of powder morphology on sintering kinetics, microstructure and mechanical properties of monazite ceramics. J. Eur. Ceram. Soc. 2018, 38, 227–234. [Google Scholar] [CrossRef]
- Li, F.T.; Ran, J.R.; Jaroniec, M.; Qiao, S.Z. Solution combustion synthesis of metal oxide nanomaterials for energy storage and conversion. Nanoscale 2015, 7, 17590. [Google Scholar] [CrossRef] [PubMed]
- Wickham, D.G. Solid-Phase Equilibria in the System NiO-Mn2O3-O2. J. Inorg. Nucl. Chem. 1964, 26, 1369–1377. [Google Scholar] [CrossRef]
- Tang, X.; Manthiram, A.; Goodenough, J. NiMn2O4 Revisited. J. Less Common Met. 1989, 156, 357–368. [Google Scholar]
- Chanel, C.; Fritsch, S.; Rousset, A.; Legros, R. Controlled Morphology of Nickel Manganite Powders. Key Eng. Mater. 1997, 132–136, 109–112. [Google Scholar] [CrossRef]
- Vidales, J.L.M.; Garcia-Chain, P.; Rojas, R.M.; Vila, E.; Martinez, O.G. Preparation and characterization of spinel type Mn-Ni-Co-O negative temperature coefficient ceramic thermistors. J. Mater. Sci. 1998, 33, 1491–1496. [Google Scholar] [CrossRef]
- Savić, S.M.; Mančić, L.; Vojisavljević, K.; Stojanović, G.; Branković, Z.; Aleksić, O.S.; Branković, G. Microstructural and electrical changes in nickel manganite powder induced by mechanical activation. Mater. Res. Bull. 2011, 46, 1065–1071. [Google Scholar] [CrossRef]
- Fang, D.; Lee, C.G.; Koo, B.H. Preparation of ultra-fine FeNiMnO4 powders and ceramics by a solid-state coordination reaction. Met. Mater. Int. 2007, 13, 165–170. [Google Scholar] [CrossRef]
- Zheng, C.-H.; Fang, D.-L. Preparation of ultra-fine cobalt-nickel manganite powders and ceramics derived from mixed oxalate. Mater. Res. Bull. 2008, 43, 1877–1882. [Google Scholar] [CrossRef]
- Le, D.T.; Jeon, C.J.; Lee, K.W.; Jeong, Y.H.; Yun, J.S.; Yoon, D.H.; Cho, J.H. Liquid flow deposited spinel (Ni, Mn)3O4 thin films for microbolometer applications. Appl. Surf. Sci. 2015, 330, 366–373. [Google Scholar] [CrossRef]
- Le, D.T.; Cho, J.H.; Ju, H. Characterization of Cu-doped (Ni, Mn)3O4 thin films annealed at low temperatures. J. Asian Ceram. Soc. 2020, 8, 814–826. [Google Scholar] [CrossRef]
- Le, D.T.; Cho, J.H.; Ju, H. Annealing temperature dependent structural and electrical properties of (Ni, Mn)3O4 thin films. Ceram. Int. 2020, 46, 25536–25545. [Google Scholar] [CrossRef]
- Shashidharagowda, H.; Mathad, S.N. Effect of incorporation of copper on structural properties of spinel nickel manganites by co-precipitation method. Mater. Sci. Energy Technol. 2020, 3, 201–208. [Google Scholar] [CrossRef]
- Jadhav, R.; Mathad, S.; Puri, V. Studies on the properties of Ni0.6Cu0.4Mn2O4NTC ceramic due to Fe doping. Ceram. Int. 2012, 38, 5181–5188. [Google Scholar] [CrossRef]
- Park, K.R.; Mhin, S.; Han, H.; Kim, K.M.; Shim, K.B.; Lee, J.I.; Ryu, J.H. Electrical properties of Fe doped Ni-Mn-Co-O cubic spinel nanopowders for temperature sensors. J. Ceram. Process Res. 2017, 18, 247–251. [Google Scholar]
- Abel, M.J.; Pramothkumar, A.; Archana, V.; Senthilkumar, N.; Jothivenkatachalam, K.; Prince, J.J. Facile synthesis of solar light active spinel nickel manganite (NiMn2O4) by co-precipitation route for photocatalytic application. Res. Chem. Intermed. 2020, 46, 3509–3525. [Google Scholar] [CrossRef]
- Ma, C.; Gao, H. Preparation and characterization of single-phase NiMn2O4 NTC ceramics by two-step sintering method. J. Mater. Sci. Mater. Electron. 2017, 28, 6699–6703. [Google Scholar] [CrossRef]
- Cui, M.-M.; Zhang, X.; Liu, K.-G.; Li, H.-B.; Gao, M.-M.; Liang, S. Fabrication of nano-grained negative temperature coefficient thermistors with high electrical stability. Rare Met. 2019, 1–6. [Google Scholar] [CrossRef]
- Wang, B.; Wang, J.; Chang, A.; Yao, J. Effects of particle sizes of starting oxides on the properties of spinel-type Mn1.1Co1.5Fe0.4O4 negative temperature coefficient ceramics. Ceram. Int. 2021, 47, 2531–2537. [Google Scholar] [CrossRef]
- Wang, B.; Wang, J.; Shang, D.; Chang, A.; Yao, J. Sintering temperature and XPS analysis of Co2.77Mn1.71Fe1.10Zn0.42O8 NTC ceramics. Mater. Chem. Phys. 2020, 239, 122098. [Google Scholar] [CrossRef]
- Yang, T.; Zhang, B.; Zhao, Q.; He, D.L.; Luo, P.; Chang, A. Vacuum hot pressed highly dense, nanograined Mg(Al1–xCrx)2O4 ceramics. Mater. Lett. 2017, 194, 42–44. [Google Scholar] [CrossRef]
- Sinha, A.P.B.; Sanjana, N.R.; Biswas, A.B. On the Structure of Some Manganites. ActaCrystallogr. 1957, 10, 439–440. [Google Scholar]
- Cocke, D.L.; Vepřek, S. First direct evidence of a solid state charge transfer redox system Cu2+ + Mn3+⇋ Cu+ + Mn4+ in copper manganese oxide. Solid State Commun. 1986, 57, 745–748. [Google Scholar] [CrossRef]
- Van Everbroeck, T.; Ciocarlan, R.-G.; Van Hoey, W.; Mertens, M.; Cool, P. Copper-Containing Mixed Metal Oxides (Al, Fe, Mn) for Application in Three-Way Catalysis. Catalysts 2020, 10, 1344. [Google Scholar] [CrossRef]
- Huang, Z.; Zhou, W.; Ouyang, C.; Wu, J.; Zhang, F.; Huang, J.; Gao, Y.; Chu, J. High performance of Mn-Co-Ni-O spinel nanofilms sputtered from acetate precursors. Sci. Rep. 2015, 5, srep10899. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, J.-F.; Fang, D.-L.; Wang, Z.-B.; Yang, P.-H.; Chen, C.-S. Preparation and electrical properties of copper-nickel manganite ceramic derived from mixed oxalate. Sens. Actuators A Phys. 2007, 135, 472–475. [Google Scholar] [CrossRef]
- Zhao, M.; Chen, W.; Wu, W.; Zhang, M.; Li, Z. Aging characteristic of Cu-doped nickel manganite NTC ceramics. J. Mater. Sci. Mater. Electron. 2020, 31, 11784–11790. [Google Scholar] [CrossRef]
- Fritsch, S.; Sarrias, J.; Brieu, M.; Couderc, J.J.; Baudour, J.L.; Snoeck, E.; Rousset, A. Correlation between the structure, the microstructure and the electrical properties of nickel manganite negative temperature coefficient (NTC) thermistors. Solid State Ion. 1998, 109, 229–237. [Google Scholar] [CrossRef]
- Schubert, M.; Münch, C.; Schuurman, S.; Poulain, V.; Kita, J.; Moos, R. Thermal Treatment of Aerosol Deposited NiMn2O4 NTC Thermistors for Improved Aging Stability. Sensors 2018, 18, 3982. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gillot, B.; Buguet, S.; Kester, E.; Baubet, C.; Tailhades, P. Cation valencies and distribution in the spinels CoxCuyMnzFeuO4+δ (δ ≥ 0) thin films studied by X-ray photoelectron spectroscopy. Thin Solid Films 1999, 357, 223–231. [Google Scholar] [CrossRef]
- Zhao, C.H.; Wang, B.Y.; Yang, P.H.; Winnubst, L.; Chen, C.S. Effects of Cu and Zn co-doping on the electrical properties of Ni0.5Mn2.5O4NTC ceramics. J. Eur. Ceram. Soc. 2008, 28, 35–40. [Google Scholar] [CrossRef]
- Huang, C.; Chen, L.; Zhang, Q.; Chang, S.; Zhang, B.; Chang, A.; Zhang, H. Preparation and characterization of LaMn0.5Co0.5O3–Ni0.66Mn2.34O4 composite NTC ceramics. J. Mater. Sci. Mater. Electron. 2016, 27, 7560–7565. [Google Scholar] [CrossRef]
- Lee, B.W. Preparation and characterization of spinel LiCoxMn2−xO4 by oxalate precipitation. J. Power Sources 2002, 109, 220–226. [Google Scholar] [CrossRef]
- Gao, H.; Ma, C.; Sun, B. Preparation and characterization of NiMn2O4 negative temperature coefficient ceramics by solid-state coordination reaction. J. Mater. Sci. Mater. Electron. 2014, 25, 3990–3995. [Google Scholar] [CrossRef]
- Xu, H.Y.; Le Xu, S.; Li, X.D.; Wang, H.; Yan, H. Chemical bath deposition of hausmannite Mn3O4 thin films. Appl. Surf. Sci. 2006, 252, 4091–4096. [Google Scholar] [CrossRef]








| Elements | Chemical Composition | ||
|---|---|---|---|
| Solution | As-Repared | Calcined at 650 °C | |
| Cu | 0.30 | 0.33 | 0.32 |
| Ni | 0.66 | 0.67 | 0.63 |
| Mn | 2.04 | 2.00 | 2.05 |
| Sintering Methods | T1 (°C) | Soaking Time (min) | T2(°C) | Soaking Time (min) |
|---|---|---|---|---|
| SS 1 | 1100 | 240 | ||
| TS 2 | 1200 | 10 | 900 | 240 |
| Samples | Peak Intensity (%) | Mn3+/Mn4+ Ratio | ||
|---|---|---|---|---|
| Mn2+ | Mn3+ | Mn4+ | ||
| S1 | 22.12 | 38.05 | 39.83 | 0.955 |
| S2 | 18.45 | 40.23 | 41.32 | 0.973 |
| Samples | ρ25 (Ω·cm) | B25/85 (K) | ΔR/R (%) |
|---|---|---|---|
| S1 | 67 ± 3 | 2843 ± 2 | 5.65 ± 0.2 |
| S2 | 116 ± 3 | 3012 ± 2 | 3.32 ± 0.2 |
| Samples | Binding Energy (eV) | Peak Intensity (Area %) | ||||
|---|---|---|---|---|---|---|
| Cu+ (A) | Cu2+ (B) | Cu2+(A) | Cu+ (A) | Cu2+ (B) | Cu2+ (A) | |
| Pristine | 930.2 | 933.2 | 933.9 | 16.29 | 41.56 | 42.15 |
| Aged | 930.1 | 933.2 | 934.1 | 14.68 | 39.54 | 45.78 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Le, D.T.; Ju, H. Solution Synthesis of Cubic Spinel Mn–Ni–Cu–O Thermistor Powder. Materials 2021, 14, 1389. https://doi.org/10.3390/ma14061389
Le DT, Ju H. Solution Synthesis of Cubic Spinel Mn–Ni–Cu–O Thermistor Powder. Materials. 2021; 14(6):1389. https://doi.org/10.3390/ma14061389
Chicago/Turabian StyleLe, Duc Thang, and Heongkyu Ju. 2021. "Solution Synthesis of Cubic Spinel Mn–Ni–Cu–O Thermistor Powder" Materials 14, no. 6: 1389. https://doi.org/10.3390/ma14061389