Polydopamine Ultrathin Film Growth on Mica via In-Situ Polymerization of Dopamine with Applications for Silver-Based Antimicrobial Coatings
Abstract
:1. Introduction
2. Materials and Methods
2.1. Deposition of Polydopamine (PDA) Nanofilm on Mica
2.2. Deposition of Ag NPs/Polydopamine Nanofilm on a Surgical Blade
2.3. Bacterial Growth Activity
2.4. Characterization
3. Results
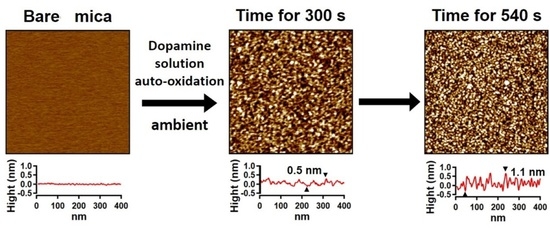
3.1. AFM Topography of PDA Film
3.2. Surface Coverage
3.3. Water Contact Angle
3.4. XPS Analysis
3.5. Antibacterial Properties of Ag/PDA Composite
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A






References
- Dang, Y.; Xing, C.-M.; Quan, M.; Wang, Y.-B.; Zhang, S.-P.; Shi, S.; Gong, Y.-K. Substrate independent coating formation and anti-biofouling performance improvement of mussel inspired polydopamine. J. Mater. Chem. B 2015, 3, 4181–4190. [Google Scholar] [CrossRef]
- Zhang, C.; Wu, B.-H.; Du, Y.; Ma, M.-Q.; Xu, Z.-K. Mussel-inspired polydopamine coatings for large-scale and angle-independent structural colors. J. Mater. Chem. C 2017, 5, 3898–3902. [Google Scholar] [CrossRef]
- Yang, W.; Liu, C.; Chen, Y. Stability of Polydopamine Coatings on Gold Substrates Inspected by Surface Plasmon Resonance Imaging. Langmuir 2018, 34, 3565–3571. [Google Scholar] [CrossRef]
- Barclay, T.G.; Hegab, H.M.; Clarke, S.R.; Ginic-Markovic, M. Versatile Surface Modification Using Polydopamine and Related Polycatecholamines: Chemistry, Structure, and Applications. Adv. Mater. Interfaces 2017, 4, 1601192. [Google Scholar] [CrossRef]
- Lee, H.; Dellatore, S.M.; Miller, W.M.; Messersmith, P.B. Mussel-Inspired Surface Chemistry for Multifunctional Coatings. Science 2007, 318, 426–430. [Google Scholar] [CrossRef] [Green Version]
- Waite, J.H. Surface chemistry: Mussel power. Nat. Mater. 2008, 7, 8–9. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.H.; Messersmith, P.B.; Lee, H. Polydopamine Surface Chemistry: A Decade of Discovery. ACS Appl. Mater. Interfaces 2018, 10, 7523–7540. [Google Scholar] [CrossRef] [PubMed]
- Nirasay, S.; Badia, A.; LeClair, G.; Claverie, J.P.; Marcotte, I. Polydopamine-Supported Lipid Bilayers. Materials 2012, 5, 2621–2636. [Google Scholar] [CrossRef] [Green Version]
- Gao, J.; Gu, H.; Xu, B. Multifunctional magnetic nanoparticles: Design, synthesis, and biomedical applications. Acc. Chem. Res. 2009, 42, 1097–1107. [Google Scholar] [CrossRef]
- Sileika, T.S.; Kim, H.-D.; Maniak, P.; Messersmith, P.B. Antibacterial performance of polydopamine-modified polymer sur-faces containing passive and active components. ACS Appl. Mater. Interfaces 2011, 3, 4602–4610. [Google Scholar] [CrossRef]
- Lee, H.; Rho, J.; Messersmith, P.B. Facile Conjugation of Biomolecules onto Surfaces via Mussel Adhesive Protein Inspired Coatings. Adv. Mater. 2009, 21, 431–434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ku, S.H.; Ryu, J.; Hong, S.K.; Lee, H.; Park, C.B. General functionalization route for cell adhesion on non-wetting surfaces. Biomaterials 2010, 31, 2535–2541. [Google Scholar] [CrossRef] [PubMed]
- d’Ischia, M.; Napolitano, A.; Pezzella, A.; Meredith, P.; Sarna, T. Chemical and Structural Diversity in Eumelanins: Unexplored Bio-Optoelectronic Materials. Angew. Chem. Int. Ed. 2009, 48, 3914–3921. [Google Scholar] [CrossRef] [PubMed]
- McCloskey, B.D.; Park, H.B.; Ju, H.; Rowe, B.W.; Miller, D.J.; Chun, B.J.; Kin, K.; Freeman, B.D. Influence of polydopamine deposition conditions on pure water flux and foulant adhesion resistance of reverse osmosis, ultrafiltration, and microfiltration membranes. Polymers 2010, 51, 3472–3485. [Google Scholar] [CrossRef]
- McCloskey, B.D.; Park, H.B.; Ju, H.; Rowe, B.W.; Miller, D.J.; Freeman, B.D. A bioinspired fouling-resistant surface modification for water purification membranes. J. Membr. Sci. 2012, 413, 82–90. [Google Scholar] [CrossRef]
- Liu, Y.; Ai, K.; Lu, L. Polydopamine and Its Derivative Materials: Synthesis and Promising Applications in Energy, Environmental, and Biomedical Fields. Chem. Rev. 2014, 114, 5057–5115. [Google Scholar] [CrossRef]
- Ou, J.; Wang, J.; Liu, S.; Zhou, J.; Yang, S. Self-Assembly and Tribological Property of a Novel 3-Layer Organic Film on Silicon Wafer with Polydopamine Coating as the Interlayer. J. Phys. Chem. C 2009, 113, 20429–20434. [Google Scholar] [CrossRef]
- Bernsmann, F.; Ponche, A.; Ringwald, C.; Hemmerlé, J.; Raya, J.; Bechinger, B.; Voegel, J.-C.; Schaaf, P.; Ball, V. Characterization of dopamine—melanin growth on silicon oxide. J. Phys. Chem. C 2009, 113, 8234–8242. [Google Scholar] [CrossRef]
- Bernsmann, F.; Ball, V.; Addiego, F.; Ponche, A.; Michel, M.; Gracio, J.J.D.A.; Toniazzo, V.; Ruch, D. Dopamine−Melanin Film Deposition Depends on the Used Oxidant and Buffer Solution. Langmuir 2011, 27, 2819–2825. [Google Scholar] [CrossRef]
- Kim, H.W.; McCloskey, B.D.; Choi, T.H.; Lee, C.; Kim, M.-J.; Freeman, B.D.; Park, H.B. Oxygen Concentration Control of Dopamine-Induced High Uniformity Surface Coating Chemistry. ACS Appl. Mater. Interfaces 2013, 5, 233–238. [Google Scholar] [CrossRef]
- Zangmeister, R.A.; Morris, T.A.; Tarlov, M.J. Characterization of polydopamine thin films deposited at short times by autoxidation of dopamine. Langmuir 2013, 29, 8619–8628. [Google Scholar] [CrossRef] [PubMed]
- Vaish, A.; Vanderah, D.J.; Richter, L.J.; Dimitriou, M.; Steffens, K.L.; Walker, M.L. Dithiol-based modification of poly(dopamine): Enabling protein resistance via short-chain ethylene oxide oligomers. Chem. Commun. 2015, 51, 6591–6594. [Google Scholar] [CrossRef] [PubMed]
- Mallinson, D.; Mullen, A.B.; Lamprou, D.A. Probing polydopamine adhesion to protein and polymer films: Microscopic and spectroscopic evaluation. J. Mater. Sci. 2018, 53, 3198–3209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alfieri, M.L.; Panzella, L.; Oscurato, S.L.; Salvatore, M.; Avolio, R.; Errico, M.E.; Maddalena, P.; Napolitano, A.; D’Ischia, M. The Chemistry of Polydopamine Film Formation: The Amine-Quinone Interplay. Biomimetics 2018, 3, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, C.; Gong, L.; Xiang, L.; Du, Y.; Hu, W.; Zeng, H.; Xu, Z.-K. Deposition and Adhesion of Polydopamine on the Surfaces of Varying Wettability. ACS Appl. Mater. Interfaces 2017, 9, 30943–30950. [Google Scholar] [CrossRef]
- Cruickshank, L.; Kennedy, A.R.; Shankland, N. Tautomeric and ionisation forms of dopamine and tyramine in the solid state. J. Mol. Struct. 2013, 1051, 132–136. [Google Scholar] [CrossRef]
- Chen, C.-T.; Martin-Martinez, F.J.; Jung, G.S.; Buehler, M.J. Polydopamine and eumelanin molecular structures investigated with ab initio calculations. Chem. Sci. 2017, 8, 1631–1641. [Google Scholar] [CrossRef] [Green Version]
- Dobbs, H.A.; Kaufman, Y.; Scott, J.; Kristiansen, K.; Schrader, A.M.; Chen, S.-Y.; Duda, P., III; Israelachvili, J.N. Ultra-Smooth, Chemically Functional Silica Surfaces for Surface Interaction Measurements and Optical/Interferometry-Based Techniques. Adv. Eng. Mater. 2018, 20, 1700630. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, H.; Huo, K.; Cui, L.; Zhang, W.; Ni, H.; Zhang, Y.; Wu, Z.; Chu, P.K. Antibacterial nano-structured titania coating incorporated with silver nanoparticles. Biomaterials 2011, 32, 5706–5716. [Google Scholar] [CrossRef]
- Yin, I.X.; Zhang, J.; Zhao, I.S.; Mei, M.L.; Li, Q.; Chu, C.H. The Antibacterial Mechanism of Silver Nanoparticles and Its Application in Dentistry. Int. J. Nanomed. 2020, 15, 2555–2562. [Google Scholar] [CrossRef] [Green Version]
- Zhou, H.; Liu, Y.; Chi, W.; Yu, C.; Yu, Y. Preparation and antibacterial properties of Ag@polydopamine/graphene oxide sheet nanocomposite. Appl. Surf. Sci. 2013, 282, 181–185. [Google Scholar] [CrossRef]
- Shalev, T.; Gopin, A.; Bauer, M.; Stark, R.W.; Rahimipour, S. Non-leaching antimicrobial surfaces through polydopamine bio-inspired coating of quaternary ammonium salts or an ultrashort antimicrobial lipopeptide. J. Mater. Chem. 2012, 22, 2026–2032. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Yu, B.; Hao, J.; Zhou, F. Amination of surfaces via self-assembly of dopamine. J. Coll. Interface Sci. 2011, 362, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Floro, J.A.; Hearne, S.J.; Hunter, J.A.; Kotula, P.; Chason, E.; Seel, S.C.; Thompson, C.V. The dynamic competition between stress generation and relaxation mechanisms during coalescence of Volmer–Weber thin films. J. Appl. Phys. 2001, 89, 4886–4897. [Google Scholar] [CrossRef] [Green Version]
- Dreyer, D.R.; Miller, D.J.; Freeman, B.D.; Paul, D.R.; Bielawski, C.W. Perspectives on poly(dopamine). Chem. Sci. 2013, 4, 3796–3802. [Google Scholar] [CrossRef]
- Jiang, J.; Zhu, L.; Zhu, L.; Zhu, B.; Xu, Y. Surface Characteristics of a Self-Polymerized Dopamine Coating Deposited on Hy-drophobic Polymer Films. Langmuir 2011, 27, 14180–14187. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.J.; Cai, T.; Neoh, K.G.; Kang, E.-T.; Dickinson, G.H.; Teo, S.L.-M.; Rittschof, D. Biomimetic Anchors for Antifouling and Antibacterial Polymer Brushes on Stainless Steel. Langmuir 2011, 27, 7065–7076. [Google Scholar] [CrossRef]
- Xuan, Y.; Jiang, G.; Li, Y.; Wang, J.; Geng, H. Inhibiting effect of dopamine adsorption and polymerization on hydrated swelling of montmorillonite. Coll. Surf. A Physicochem. Eng. Asp. 2013, 422, 50–60. [Google Scholar] [CrossRef]
- Dreyer, D.R.; Miller, D.J.; Freeman, B.D.; Paul, D.R.; Bielawski, C.W. Elucidating the Structure of Poly(dopamine). Langmuir 2012, 28, 6428–6435. [Google Scholar] [CrossRef]
- Kung, M.-L.; Lin, P.-Y.; Peng, S.-W.; Wu, D.-C.; Wu, W.-J.; Yeh, B.-W.; Tai, M.-H.; Hung, H.-S.; Hsieh, S. Biomimetic polymer-based Ag nanocomposites as a antimicrobial platform. Appl. Mater. Today 2016, 4, 31–39. [Google Scholar] [CrossRef]






| Time (s) | 0 | 60 | 90 | 120 | 150 |
|---|---|---|---|---|---|
| RMS (nm) | 21.4 a | 117.9 b | 158.6 c | 163.8 c,d,i | 197.9 e |
| contact angle (°) | 14.5 ± 0.9 a | 15.8 ± 0.1 a | 22.3 ± 0.6 b | 28.4 ± 2.4 c | 33.4 ± 0.3 d |
| Time (s) | 180 | 210 | 300 | 420 | 540 |
| RMS (nm) | 273.4 f | 266.6 d,f,g | 242.1 h | 171.4 i | 250.2 g,h |
| contact angle (°) | 37.4 ± 0.9 e | 42.6 ± 2.2 f | 47.8 ± 0.7 g | 48.1 ± 0.6 g,h | 49.9 ± 1.1 h |
| Time (s) | Composition (%) | |||||
|---|---|---|---|---|---|---|
| CH and C–NH2 | C–OH and C–N | C=O | =N–R | R2–NH | R–NH2 | |
| 60 | 50.0 | 46.9 | 3.1 | 3.0 | 44.6 | 52.4 |
| 150 | 43.1 | 49.3 | 7.6 | 3.7 | 52.2 | 44.1 |
| 300 | 46.5 | 43.8 | 9.7 | 6.1 | 50.1 | 43.8 |
| 540 | 42.1 | 42.7 | 15.2 | 8.5 | 70.5 | 21.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Z.-H.; Peng, S.-W.; Hsieh, S.-L.; Kirankumar, R.; Huang, P.-F.; Chang, T.-M.; Dwivedi, A.K.; Chen, N.-F.; Wu, H.-M.; Hsieh, S. Polydopamine Ultrathin Film Growth on Mica via In-Situ Polymerization of Dopamine with Applications for Silver-Based Antimicrobial Coatings. Materials 2021, 14, 671. https://doi.org/10.3390/ma14030671
Huang Z-H, Peng S-W, Hsieh S-L, Kirankumar R, Huang P-F, Chang T-M, Dwivedi AK, Chen N-F, Wu H-M, Hsieh S. Polydopamine Ultrathin Film Growth on Mica via In-Situ Polymerization of Dopamine with Applications for Silver-Based Antimicrobial Coatings. Materials. 2021; 14(3):671. https://doi.org/10.3390/ma14030671
Chicago/Turabian StyleHuang, Zheng-Hao, Shi-Wei Peng, Shu-Ling Hsieh, Rajendranath Kirankumar, Po-Feng Huang, Tsao-Ming Chang, Atul Kumar Dwivedi, Nan-Fu Chen, Hao-Ming Wu, and Shuchen Hsieh. 2021. "Polydopamine Ultrathin Film Growth on Mica via In-Situ Polymerization of Dopamine with Applications for Silver-Based Antimicrobial Coatings" Materials 14, no. 3: 671. https://doi.org/10.3390/ma14030671