Hybrid Biocomposites Based on Poly(Lactic Acid) and Silica Aerogel for Food Packaging Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Film Samples Preparation
2.3. Thermal Characterization
2.3.1. Differential Scanning Calorimetry (DSC)
2.3.2. Thermogravimetric Analysis (TGA)
2.4. Mechanical Characterization
2.5. Scanning Electron Microscopy (SEM)
2.6. Permeability to Gases
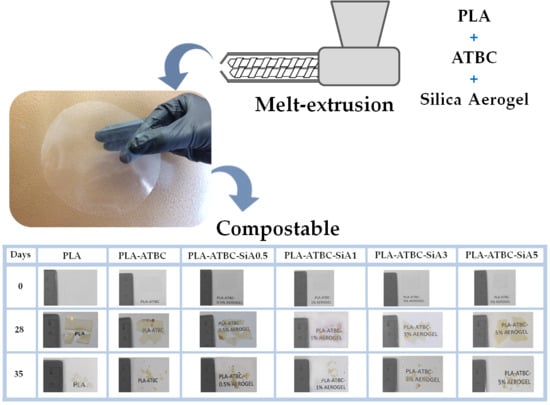
2.7. Disintegrability under Composting Conditions
3. Results
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Guo, X.; Xiang, D.; Duan, G.; Mou, P. A review of mechanochemistry applications in waste management. Waste Manag. 2010, 30, 4–10. [Google Scholar] [CrossRef]
- Arrieta, M.; Samper, M.; Aldas, M.; López, J. On the use of PLA-PHB blends for sustainable food packaging applications. Materials 2017, 10, 1008. [Google Scholar] [CrossRef]
- Auras, R.; Harte, B.; Selke, S. An overview of polylactides as packaging materials. Macromol. Biosci. 2004, 4, 835–864. [Google Scholar] [CrossRef]
- Armentano, I.; Bitinis, N.; Fortunati, E.; Mattioli, S.; Rescignano, N.; Verdejo, R.; Lopez-Manchado, M.A.; Kenny, J.M. Multifunctional nanostructured pla materials for packaging and tissue engineering. Prog. Polym. Sci. 2013, 38, 1720–1747. [Google Scholar] [CrossRef] [Green Version]
- Lim, L.T.; Auras, R.; Rubino, M. Processing technologies for poly(lactic acid). Prog. Polym. Sci. (Oxf.) 2008, 33, 820–852. [Google Scholar] [CrossRef]
- Arrieta, M.P.; López, J.; López, D.; Kenny, J.M.; Peponi, L. Development of flexible materials based on plasticized electrospun pla–phb blends: Structural, thermal, mechanical and disintegration properties. Eur. Polym. J. 2015, 73, 433–446. [Google Scholar] [CrossRef]
- Arrieta, M.P.; Peponi, L.; López, D.; Fernández-García, M. Recovery of yerba mate (Ilex paraguariensis) residue for the development of PLA-based bionanocomposite films. Ind. Crop. Prod. 2018, 111, 317–328. [Google Scholar] [CrossRef]
- Kale, G.; Kijchavengkul, T.; Auras, R.; Rubino, M.; Selke, S.E.; Singh, S.P. Compostability of bioplastic packaging materials: An overview. Macromol. Biosci. 2007, 7, 255–277. [Google Scholar] [CrossRef]
- Fortunati, E.; Armentano, I.; Iannoni, A.; Barbale, M.; Zaccheo, S.; Scavone, M.; Visai, L.; Kenny, J. New Multifunctional Poly(Lactide Acid) Composites: Mechanical, Antibacterial, and Degradation Properties; Wiley: Hoboken, NJ, USA, 2012; Volume 124. [Google Scholar]
- Gonçalves de Moura, I.; Vasconcelos de Sá, A.; Lemos Machado Abreu, A.S.; Alves Machado, A.V. 7—Bioplastics from agro-wastes for food packaging applications a2—Grumezescu, alexandru mihai. In Food Packaging; Academic Press: Cambridge, MA, USA, 2017; pp. 223–263. [Google Scholar]
- Burgos, N.; Martino, V.P.; Jiménez, A. Characterization and ageing study of poly (lactic acid) films plasticized with oligomeric lactic acid. Polym. Degrad. Stab. 2013, 98, 651–658. [Google Scholar] [CrossRef]
- Arrieta, M.P.; Fortunati, E.; Dominici, F.; López, J.; Kenny, J.M. Bionanocomposite films based on plasticized PLA–PHB/cellulose nanocrystal blends. Carbohydr. Polym. 2015, 121, 265–275. [Google Scholar] [CrossRef] [Green Version]
- Ferri, J.M.; Samper, M.D.; García-Sanoguera, D.; Reig, M.J.; Fenollar, O.; Balart, R. Plasticizing effect of biobased epoxidized fatty acid esters on mechanical and thermal properties of poly(lactic acid). J. Mater. Sci. 2016, 51, 5356–5366. [Google Scholar] [CrossRef]
- Courgneau, C.; Domenek, S.; Guinault, A.; Avérous, L.; Ducruet, V. Analysis of the structure-properties relationships of different multiphase systems based on plasticized poly (lactic acid). J. Polym. Environ. 2011, 19, 362–371. [Google Scholar] [CrossRef]
- Arrieta, M.P.; López, J.; López, D.; Kenny, J.M.; Peponi, L. Effect of chitosan and catechin addition on the structural, thermal, mechanical and disintegration properties of plasticized electrospun PLA-PHB biocomposites. Polym. Degrad. Stab. 2016, 132, 145–156. [Google Scholar] [CrossRef]
- Labrecque, L.; Kumar, R.A.; Davé, V.; Gross, R.A.; McCarthy, S. Citrate esters as plasticizers for poly(lactic acid). J. Appl. Polym. Sci. 1997, 66, 1507–1513. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, J. Research progress in toughening modification of poly(lactic acid). J. Polym. Sci. Part B Polym. Phys. 2011, 49, 1051–1083. [Google Scholar] [CrossRef]
- Arrieta, M.P.; López de Dicastillo, C.; Garrido, L.; Roa, K.; Galotto, M.J. Electrospun pva fibers loaded with antioxidant fillers extracted from durvillaea antarctica algae and their effect on plasticized PLA bionanocomposites. Eur. Polym. J. 2018, 103, 145–157. [Google Scholar] [CrossRef]
- Aliotta, L.; Vannozzi, A.; Panariello, L.; Gigante, V.; Coltelli, M.-B.; Lazzeri, A. Sustainable micro and nano additives for controlling the migration of a biobased plasticizer from pla-based flexible films. Polymers 2020, 12, 1366. [Google Scholar] [CrossRef]
- Courgneau, C.; Ducruet, V.; Avérous, L.; Grenet, J.; Domenek, S. Nonisothermal crystallization kinetics of poly(lactide)—Effect of plasticizers and nucleating agent. Polym. Eng. Sci. 2013, 53, 1085–1098. [Google Scholar] [CrossRef]
- Arrieta, M.P.; Samper, M.D.; López, J.; Jiménez, A. Combined effect of poly(hydroxybutyrate) and plasticizers on polylactic acid properties for film intended for food packaging. J. Polym. Environ. 2014, 22, 460–470. [Google Scholar] [CrossRef]
- European Food Safety Authority. Scientific opinion on flavouring group evaluation 10, revision 3 (fge.10rev3): Aliphatic primary and secondary saturated and unsaturated alcohols, aldehydes, acetals, carboxylic acids and esters containing an additional oxygenated functional group and lactones from chemical groups 9, 13 and 30. EFSA J. 2012, 10, 25–63. [Google Scholar]
- Arrieta, M.P.; López, J.; Hernández, A.; Rayón, E. Ternary PLA–PHB–limonene blends intended for biodegradable food packaging applications. Eur. Polym. J. 2014, 50, 255–270. [Google Scholar] [CrossRef]
- Arrieta, M.P.; Sessini, V.; Peponi, L. Biodegradable poly(ester-urethane) incorporated with catechin with shape memory and antioxidant activity for food packaging. Eur. Polym. J. 2017, 94, 111–124. [Google Scholar] [CrossRef]
- Navarro-Baena, I.; Arrieta, M.P.; Sonseca, A.; Torre, L.; López, D.; Giménez, E.; Kenny, J.M.; Peponi, L. Biodegradable nanocomposites based on poly(ester-urethane) and nanosized hydroxyapatite: Plastificant and reinforcement effects. Polym. Degrad. Stab. 2015, 121, 171–179. [Google Scholar] [CrossRef]
- Bao, L.; Dorgan, J.R.; Knauss, D.; Hait, S.; Oliveira, N.S.; Maruccho, I.M. Gas permeation properties of poly(lactic acid) revisited. J. Membr. Sci. 2006, 285, 166–172. [Google Scholar] [CrossRef]
- Burgos, N.; Armentano, I.; Fortunati, E.; Dominici, F.; Luzi, F.; Fiori, S.; Cristofaro, F.; Visai, L.; Jiménez, A.; Kenny, J.M. Functional properties of plasticized bio-based poly(lactic acid)_poly(hydroxybutyrate) (PLA_PHB) films for active food packaging. Food Bioprocess Technol. 2017, 10, 770–780. [Google Scholar] [CrossRef] [Green Version]
- Venkata Prasad, C.; Sudhakara, P.; Prabhakar, M.N.; Ur Rehman Shah, A.; Song, J.I. An investigation on the effect of silica aerogel content on thermal and mechanical properties of sisal/pla nano composites. Polym. Compos. 2018, 39, 835–840. [Google Scholar] [CrossRef]
- Salgado, C.; Arrieta, M.P.; Peponi, L.; López, D.; Fernández-García, M. Photo-crosslinkable polyurethanes reinforced with coumarin modified silica nanoparticles for photo-responsive coatings. Prog. Org. Coat. 2018, 123, 63–74. [Google Scholar] [CrossRef]
- Choi, H.; Parale, V.G.; Lee, K.-Y.; Nah, H.-Y.; Driss, Z.; Driss, D.; Bouabidi, A.; Euchy, S.; Park, H.-H. Polypropylene/silica aerogel composite incorporating a conformal coating of methyltrimethoxysilane-based aerogel. J. Nanosci. Nanotechnol. 2019, 19, 1376–1381. [Google Scholar] [CrossRef]
- Zolfaghari, S.; Paydayesh, A.; Jafari, M. Mechanical and thermal properties of polypropylene/silica aerogel composites. J. Macromol. Sci. Part B 2019, 58, 305–316. [Google Scholar] [CrossRef]
- Chen, C.; Ding, R.; Yang, S.; Wang, J.; Chen, W.; Zong, L.; Xie, J. Development of thermal insulation packaging film based on poly(vinyl alcohol) incorporated with silica aerogel for food packaging application. LWT 2020, 129, 109568. [Google Scholar] [CrossRef]
- Ndazi, B.S.; Karlsson, S. Characterization of Hydrolytic Degradation of Polylactic Acid/Rice Hulls Composites in Water at Different Temperatures; University of Dar es Salaam: Dar es Salaam, Tanzania, 2011. [Google Scholar]
- Bitinis, N.; Verdejo, R.; Bras, J.; Fortunati, E.; Kenny, J.M.; Torre, L.; López-Manchado, M.A. Poly(lactic acid)/natural rubber/cellulose nanocrystal bionanocomposites part i. Processing and morphology. Carbohydr. Polym. 2013, 96, 611–620. [Google Scholar] [CrossRef]
- Murariu, M.; Doumbia, A.; Bonnaud, L.; Dechief, A.L.; Paint, Y.; Ferreira, M.; Campagne, C.; Devaux, E.; Dubois, P. High-performance polylactide/ZnO nanocomposites designed for films and fibers with special end-use properties. Biomacromolecules 2011, 12, 1762–1771. [Google Scholar] [CrossRef]
- Turner Ii, J.F.; Riga, A.; O’Connor, A.; Zhang, J.; Collis, J. Characterization of drawn and undrawn poly-l-lactide films by differential scanning calorimetry. J. Therm. Anal. Calorim. 2004, 75, 257–268. [Google Scholar] [CrossRef]
- Tiemblo, P.; Guzman, J.; Riande, E.; Salvador, E.; Peinado, C. Gas-transport properties in crosslinked polymers. I. Aliphatic polyurethane–acrylate-based adhesives. J. Polym. Sci. Part B Polym. Phys. 2001, 39, 786–795. [Google Scholar] [CrossRef]
- Vargas, J.; Santiago, A.A.; Cruz-Morales, J.A.; Tlenkopatchev, M.A.; de Lys, T.; López-González, M.; Riande, E. Gas transport properties of hydrogenated and fluorinated polynorbornene dicarboximides. Macromol. Chem. Phys. 2013, 214, 2607–2615. [Google Scholar] [CrossRef]
- UNE-EN ISO. ISO 20200:2015. Plastics. Determination of the Degree of Disintegration of Plastic Materials under Simulated Composting Conditions in a Laboratory-Scale Test; ISO: Geneva, Switzerland, 2016. [Google Scholar]
- Arrieta, M.P.; López, J.; Rayón, E.; Jiménez, A. Disintegrability under composting conditions of plasticized pla–phb blends. Polym. Degrad. Stab. 2014, 108, 307–318. [Google Scholar] [CrossRef] [Green Version]
- García-Arroyo, P.; Arrieta, M.P.; Garcia-Garcia, D.; Cuervo-Rodríguez, R.; Fombuena, V.; Mancheño, M.J.; Segura, J.L. Plasticized poly(lactic acid) reinforced with antioxidant covalent organic frameworks (COFs) as novel nanofillers designed for non-migrating active packaging applications. Polymer 2020, 196, 122466. [Google Scholar] [CrossRef]
- Muller, J.; Jiménez, A.; González-Martínez, C.; Chiralt, A. Influence of plasticizers on thermal properties and crystallization behaviour of poly (lactic acid) films obtained by compression moulding. Polym. Int. 2016, 65, 970–978. [Google Scholar] [CrossRef]
- Hauser, C.; Peñaloza, A.; Guarda, A.; Galotto, M.J.; Bruna, J.E.; Rodríguez, F.J. Development of an active packaging film based on a methylcellulose coating containing murta (Ugni molinae turcz) leaf extract. Food Bioprocess Technol. 2016, 9, 298–307. [Google Scholar] [CrossRef]
- Arrieta, M.P.; Garrido, L.; Faba, S.; Guarda, A.; Galotto, M.J.; Dicastillo, C.L.d. Cucumis metuliferus fruit extract loaded acetate cellulose coatings for antioxidant active packaging. Polymers 2020, 12, 1248. [Google Scholar] [CrossRef]
- Briassoulis, D. Analysis of the mechanical and degradation performances of optimised agricultural biodegradable films. Polym. Degrad. Stab. 2007, 92, 1115–1132. [Google Scholar] [CrossRef]
- Arrieta, M.P.; Peponi, L.; López, D.; López, J.; Kenny, J.M. An overview of nanoparticles role in the improvement of barrier properties of bioplastics for food packaging applications. In Food Packaging; Elsevier: Amsterdam, The Netherlands, 2017; pp. 391–424. [Google Scholar]
- Auras, R.A. Chapter 19—Solubility of gases and vapors in polylactide polymers. In Thermodynamics, Solubility and Environmental Issues; Letcher, T.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2007; pp. 343–368. [Google Scholar]
- Sereda, L.; López-González, M.; Yuan Visconte, L.L.; Nunes, R.C.R.; Guimarães Furtado, C.R.; Riande, E. Influence of silica and black rice husk ash fillers on the diffusivity and solubility of gases in silicone rubbers. Polymer 2003, 44, 3085–3093. [Google Scholar] [CrossRef]
- Jiang, L.; Zhang, J. 7—Biodegradable and biobased polymers a2—Kutz, myer. In Applied Plastics Engineering Handbooki, 2nd ed.; William Andrew Publishing, Myer Kutz Associates. Inc.: Delmar, NY, USA, 2017; pp. 127–143. [Google Scholar]
- Kale, G.; Auras, R.; Singh, S.P. Comparison of the degradability of poly (lactide) packages in composting and ambient exposure conditions. Packag. Technol. Sci. Int. J. 2007, 20, 49–70. [Google Scholar] [CrossRef]
- Fortunati, E.; Puglia, D.; Santulli, C.; Sarasini, F.; Kenny, J. Biodegradation of phormium tenax/poly (lactic acid) composites. J. Appl. Polym. Sci. 2012, 125, E562–E572. [Google Scholar] [CrossRef]
- Tuominen, J.; Kylmä, J.; Kapanen, A.; Venelampi, O.; Itävaara, M.; Seppälä, J. Biodegradation of lactic acid based polymers under controlled composting conditions and evaluation of the ecotoxicological impact. Biomacromolecules 2002, 3, 445–455. [Google Scholar] [CrossRef]





| Formulation | PLA (wt.%) | ATBC (wt.%) | SiA (wt.%) |
|---|---|---|---|
| PLA | 100 | 0 | 0 |
| PLA-ATBC | 85 | 15 | 0 |
| PLA-ATBC-SiA0.5% | 84.5 | 15 | 0.5 |
| PLA-ATBC-SiA1% | 84 | 15 | 1 |
| PLA-ATBC-SiA3% | 82 | 15 | 3 |
| PLA-ATBC-SiA5% | 80 | 15 | 5 |
| Film Formulations | T0 (°C) | Tmax I (°C) | Tmax II (°C) |
|---|---|---|---|
| PLA | 322 | - | 360 |
| PLA-ATBC | 205 | 217 | 361 |
| PLA-ATBC-SiA0.5% | 215 | 212 | 358 |
| PLA-ATBC- SiA1% | 204 | 215 | 360 |
| PLA-ATBC- SiA3% | 236 | 216 | 361 |
| PLA-ATBC- SiA5% | 249 | 213 | 348 |
| Film Formulations | Tg (°C) | Tcc (°C) | ∆Hcc (J g−1) | Tm (°C) | ∆Hm (J g−1) | χc (%) | ||
| First Heating Scan | ||||||||
| PLA | 59 | 109 | 30.2 | 175 | 52.0 | 22.1 | ||
| PLA-ATBC | 50 | 74 | 13.4 | 166 | 54.0 | 51.3 | ||
| PLA-ATBC-SiA0.5% | 49 | 75 | 11.9 | 166 | 51.9 | 50.9 | ||
| PLA-ATBC-SiA1% | 47 | 79 | 13.7 | 167 | 53.0 | 50.2 | ||
| PLA-ATBC-SiA3% | 48 | 75 | 10.9 | 166 | 48.3 | 49.0 | ||
| PLA-ATBC-SiA5% | 48 | 78 | 12.6 | 167 | 49.2 | 49.1 | ||
| Film Formulations | Cooling Scan | Second Heating Scan | ||||||
| Tg (°C) | Tcc (°C) | ∆Hcc (J g−1) | Tcc (°C) | ∆Hcc (J g−1) | Tm (°C) | ∆Hm (J g−1) | χc (%) | |
| PLA | 63 | 109 | 21.0 | 107 | 4.0 | 175 | 53.4 | 30.5 |
| PLA-ATBC | - | 88 | 28.5 | - | 0.6 | 167 | 46.2 | 58.4 |
| PLA-ATBC-SiA0.5% | - | 88 | 23.2 | - | - | 167 | 46.2 | 58.7 |
| PLA-ATBC-SiA1% | - | 89 | 24.3 | - | - | 168 | 46.8 | 59.9 |
| PLA-ATBC-SiA3% | - | 89 | 24.3 | - | - | 166 | 40.6 | 53.2 |
| PLA-ATBC-SiA5% | - | 87 | 25.4 | - | - | 167 | 40.3 | 54.2 |
| Film Formulation | Film Thickness (µm) | Gas | P (Barrer) | D × 108 (cm2 s−1) | S × 103 (cm3 (STP) cm−3(cm Hg)−1) |
|---|---|---|---|---|---|
| PLA | 99 | CO2 | 1.03 | 0.31 | 33.1 |
| N2 | 0.06 | 0.44 | 1.3 | ||
| O2 | 0.32 | 1.83 | 1.7 | ||
| PLA-ATBC | 185 | CO2 | 3.21 | 1.66 | 19.4 |
| N2 | 0.22 | 1.93 | 1.2 | ||
| O2 | 0.84 | 7.36 | 1.1 | ||
| PLA-ATBC-SiA0.5% | 185 | CO2 | 10.91 | 4.62 | 23.6 |
| N2 | 0.47 | 10.66 | 0.4 | ||
| O2 | 1.72 | 17.62 | 1.0 | ||
| PLA-ATBC-SiA1% | 168 | CO2 | 11.21 | 4.95 | 22.7 |
| N2 | 0.44 | 9.17 | 0.5 | ||
| O2 | 1.64 | 17.06 | 1.0 | ||
| PLA-ATBC-SiA3% | 175 | CO2 | 3.40 | 1.33 | 25.6 |
| N2 | 0.16 | 1.78 | 0.9 | ||
| O2 | 0.75 | 5.42 | 1.4 | ||
| PLA-ATBC-SiA5% | 197 | CO2 | 3.56 | 1.38 | 25.9 |
| N2 | 0.20 | 1.46 | 1.4 | ||
| O2 | 0.76 | 5.40 | 1.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aragón-Gutierrez, A.; Arrieta, M.P.; López-González, M.; Fernández-García, M.; López, D. Hybrid Biocomposites Based on Poly(Lactic Acid) and Silica Aerogel for Food Packaging Applications. Materials 2020, 13, 4910. https://doi.org/10.3390/ma13214910
Aragón-Gutierrez A, Arrieta MP, López-González M, Fernández-García M, López D. Hybrid Biocomposites Based on Poly(Lactic Acid) and Silica Aerogel for Food Packaging Applications. Materials. 2020; 13(21):4910. https://doi.org/10.3390/ma13214910
Chicago/Turabian StyleAragón-Gutierrez, Alejandro, Marina P. Arrieta, Mar López-González, Marta Fernández-García, and Daniel López. 2020. "Hybrid Biocomposites Based on Poly(Lactic Acid) and Silica Aerogel for Food Packaging Applications" Materials 13, no. 21: 4910. https://doi.org/10.3390/ma13214910