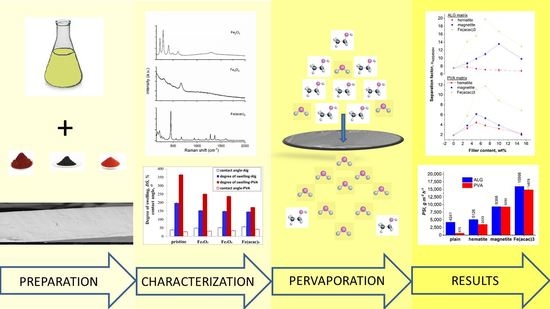
Collation Efficiency of Poly(Vinyl Alcohol) and Alginate Membranes with Iron-Based Magnetic Organic/Inorganic Fillers in Pervaporative Dehydration of Ethanol
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Preparation of Iron-Based Fillers
2.3. Membrane Fabrication
2.4. Physico-Chemical Characterization
2.5. Pervaporation Experiments
3. Results and Discussion
3.1. Membrane Characterization
3.1.1. Raman Studies
3.1.2. Swelling and Contact Angle Measurements
3.1.3. Structural Analysis of Iron-base Fillers
3.1.4. Morphological Analysis
3.2. Pervaporative Performance of Magnetic Hybrid Membranes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ahmadzadeh, M.; Romero, C.; McCloy, J. Magnetic analysis of commercial hematite, magnetite, and their mixtures. AIP Adv. 2018, 8, 56807. [Google Scholar] [CrossRef] [Green Version]
- Vane, L.M. Review: membrane materials for the removal of water from industrial solvents by pervaporation and vapor permeation. J. Chem. Technol. Biotechnol. 2018, 94, 343–365. [Google Scholar] [CrossRef] [PubMed]
- Shao, P.; Huang, R.Y.M. Polymeric membrane pervaporation. J. Memb. Sci. 2007, 287, 162–179. [Google Scholar] [CrossRef]
- Sridhar, S.; Moulik, S. Membrane Processes: Pervaporation, Vapor Permeation and Membrane Distillation for Industrial Scale Separations. Advances in Membrane Processes; Wiley: Hoboken, NJ, USA, 2018. [Google Scholar]
- Jyothi, M.; Reddy, K.R.; Soontarapa, K.; Naveen, S.; Raghu, A.V.; Kulkarni, R.V.; Suhas, D.; Shetti, N.P.; Nadagouda, M.N.; Aminabhavi, T.M. Membranes for dehydration of alcohols via pervaporation. J. Environ. Manag. 2019, 242, 415–429. [Google Scholar] [CrossRef]
- Bolto, B.; Hoang, M.; Xie, Z. A review of membrane selection for the dehydration of aqueous ethanol by pervaporation. Chem. Eng. Process. Process. Intensif. 2011, 50, 227–235. [Google Scholar] [CrossRef]
- Castro-Muñoz, R.; Galiano, F.; Figoli, A. Chemical and bio-chemical reactions assisted by pervaporation technology. Crit. Rev. Biotechnol. 2019, 39, 884–903. [Google Scholar] [CrossRef] [PubMed]
- Castro-Muñoz, R.; Boczkaj, G.; Gontarek, E.; Cassano, A.; Fíla, V. Membrane technologies assisting plant-based and agro-food by-products processing: A comprehensive review. Trends Food Sci. Technol. 2020, 95, 219–232. [Google Scholar] [CrossRef]
- Castro-Muñoz, R. Pervaporation-based membrane processes for the production of non-alcoholic beverages. J. Food Sci. Technol. 2019, 56, 2333–2344. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Pan, F.; Wang, M.; Li, W.; Song, Y.; Liu, G.; Yang, H.; Gao, B.; Wu, H.; Jiang, Z. Hybrid membranes for pervaporation separations. J. Membr. Sci. 2017, 541, 329–346. [Google Scholar] [CrossRef]
- Ong, Y.K.; Shi, G.M.; Le, N.L.; Tang, Y.P.; Zuo, J.; Nunes, S.P.; Chung, T.-S. Recent membrane development for pervaporation processes. Prog. Polym. Sci. 2016, 57, 1–31. [Google Scholar] [CrossRef] [Green Version]
- Chapman, P.D.; Oliveira, T.; Livingston, A.G.; Li, K. Membranes for the dehydration of solvents by pervaporation. J. Membr. Sci. 2008, 318, 5–37. [Google Scholar] [CrossRef]
- Souza, V.C.; Quadri, M.G.N. Organic-inorganic hybrid membranes in separation processes: a 10-year review. Braz. J. Chem. Eng. 2013, 30, 683–700. [Google Scholar] [CrossRef]
- Bowen, T.C.; Noble, R.D.; Falconer, J.L. Fundamentals and applications of pervaporation through zeolite membranes. J. Membr. Sci. 2004, 245, 1–33. [Google Scholar] [CrossRef]
- Abraham, J.; Jose, T.; Moni, G.; George, S.C.; Kalarikkal, N.; Thomas, S. Ionic liquid modified multiwalled carbon nanotube embedded styrene butadiene rubber membranes for the selective removal of toluene from toluene/methanol mixture via pervaporation. J. Taiwan Inst. Chem. Eng. 2019, 95, 594–601. [Google Scholar] [CrossRef]
- Araki, S.; Imasaka, S.; Tanaka, S.; Miyake, Y. Pervaporation of organic/water mixtures with hydrophobic silica membranes functionalized by phenyl groups. J. Membr. Sci. 2011, 380, 41–47. [Google Scholar] [CrossRef]
- Castro-Muñoz, R.; Buera-González, J.; Iglesia, Ó.; Galiano, F.; Fíla, V.; Malankowska, M.; Rubio, C.; Figoli, A.; Téllez, C.; Coronas, J. Towards the dehydration of ethanol using pervaporation cross-linked poly(vinyl alcohol)/graphene oxide membranes. J. Memb. Sci. 2019, 582, 423–434. [Google Scholar] [CrossRef] [Green Version]
- Jia, Z.; Wu, G. Metal-organic frameworks based mixed matrix membranes for pervaporation. Microporous Mesoporous Mater. 2016, 235, 151–159. [Google Scholar] [CrossRef]
- Dudek, G.; Gnus, M.; Strzelewicz, A.; Turczyn, R.; Krasowska, M. The influence of metal oxides on the separation properties of hybrid alginate membranes. Sep. Sci. Technol. 2017, 53, 1178–1190. [Google Scholar] [CrossRef]
- Tadic, M.; Panjan, M.; Damnjanovic, V.; Milosevic, I. Magnetic properties of hematite (α-Fe2O3) nanoparticles prepared by hydrothermal synthesis method. Appl. Surf. Sci. 2014, 320, 183–187. [Google Scholar] [CrossRef]
- Huang, D.J.; Chang, C.F.; Jeng, H.-T.; Guo, G.-Y.; Lin, H.-J.; Wu, W.B.; Ku, H.; Fujimori, A.; Takahashi, Y.; Chen, C.T. Spin and Orbital Magnetic Moments of Fe3O4. Phys. Rev. Lett. 2004, 93, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Lübken, D.; Saxarra, M.; Kalesse, M. Tris(acetylacetonato) Iron(III): Recent Developments and Synthetic Applications. Synth. 2018, 51, 161–177. [Google Scholar] [CrossRef] [Green Version]
- Schafer, A.I.; Fane, A.G.; Waite, T.D. Chemical Addition Prior to Membrane Processes for Natural Organic Matter (NOM) Removal. Chemical Water and Wastewater Treatment V 1998, 125–137. [Google Scholar] [CrossRef]
- Xia, C.; Afzal, M.; Wang, B.; Soltaninazarlou, A.; Zhang, W.; Cai, Y.; Zhu, B. Mixed-conductive membrane composed of natural hematite and Ni0.8Co0.15Al0.05LiO2-δ for electrolyte layer-free fuel cell. Adv. Mater. Lett. 2016, 8, 114–121. [Google Scholar] [CrossRef]
- Kang, W.; Li, F.; Zhao, Y.; Qiao, C.; Ju, J.; Cheng, B. Fabrication of porous Fe2O3/PTFE nanofiber membranes and their application as a catalyst for dye degradation. RSC Adv. 2016, 6, 32646–32652. [Google Scholar] [CrossRef]
- Bahmani, P.; Maleki, A.; Daraei, H.; Khamforoush, M.; Athar, S.D.; Gharibi, F. Fabrication and characterization of novel polyacrylonitrile/α-Fe2O3 ultrafiltration mixed-matrix membranes for nitrate removal from aqueous solutions. J. Mol. Liq. 2018, 271, 557–570. [Google Scholar] [CrossRef]
- Bahmani, P.; Maleki, A.; Daraei, H.; Rezaee, R.; Khamforoush, M.; Athar, S.D.; Gharibi, F.; Ziaee, A.H.; McKay, G. Application of modified electrospun nanofiber membranes with α-Fe2O3 nanoparticles in arsenate removal from aqueous media. Environ. Sci. Pollut. Res. 2019, 26, 21993–22009. [Google Scholar] [CrossRef]
- Agbaje, T.A.; Al-Gharabli, S.I.; Mavukkandy, M.O.; Kujawa, J.; Arafat, H.A. PVDF/magnetite blend membranes for enhanced flux and salt rejection in membrane distillation. Desalination 2018, 436, 69–80. [Google Scholar] [CrossRef]
- Woo, S.T.; Yun, T.; Kwak, S.-Y. Fouling-resistant microfiltration membrane modified with magnetite nanoparticles by reversible conjunction. Sep. Purif. Technol. 2018, 202, 299–306. [Google Scholar] [CrossRef]
- Daraei, P.; Madaeni, S.S.; Ghaemi, N.; Salehi, E.; Khadivi, M.A.; Moradian, R.; Astinchap, B. Novel polyethersulfone nanocomposite membrane prepared by PANI/Fe3O4 nanoparticles with enhanced performance for Cu(II) removal from water. J. Membr. Sci. 2012, 415, 250–259. [Google Scholar] [CrossRef]
- Salehian, P.; Chua, M.L.; Askari, M.; Shi, G.M.; Chung, T.-S.; Chung, T.-S. In situ regulation of micro-pore to design high performance polyimide membranes for pervaporation dehydration of isopropanol. J. Membr. Sci. 2015, 493, 299–310. [Google Scholar] [CrossRef]
- Khalafalla, S.; Reimers, G.W. Preparation of dilution-stable aqueous magnetic fluids. IEEE Trans. Magn. 1980, 16, 178–183. [Google Scholar] [CrossRef]
- Lassoued, A.; Lassoued, M.S.; Dkhil, B.; Gadri, A.; Ammar, S. Synthesis, structural, optical and morphological characterization of hematite through the precipitation method: Effect of varying the nature of the base. J. Mol. Struct. 2017, 1141, 99–106. [Google Scholar] [CrossRef]
- Chaudhuri, M.K.; Ghosh, S.K. Notes. Novel synthesis of tris(acetylacetonato)iron(III). J. Chem. Soc. Dalton Trans. 1983, 2, 839. [Google Scholar] [CrossRef]
- Wijmans, J.; Baker, R. The solution-diffusion model: a review. J. Membr. Sci. 1995, 107, 1–21. [Google Scholar] [CrossRef]
- Geankoplis, C.J. Transport Processes and Separation Process Principles:(Includes Unit Operations), 4th ed.; Prentice-Hall International: Upper Saddle River, NJ, USA, 2014. [Google Scholar]
- Dudek, G.; Borys, P. A Simple Methodology to Estimate the Diffusion Coefficient in Pervaporation-Based Purification Experiments. Polymers 2019, 11, 343. [Google Scholar] [CrossRef] [Green Version]
- Chernyshova, I.; Hochella Jr, M.F.; Madden, A.S. Size-dependent structural transformations of hematite nanoparticles. 1. Phase transition. Phys. Chem. Chem. Phys. 2007, 9, 1736. [Google Scholar] [CrossRef]
- Shebanova, O.N.; Lazor, P. Raman spectroscopic study of magnetite (FeFe2O4): a new assignment for the vibrational spectrum. J. Solid State Chem. 2003, 174, 424–430. [Google Scholar] [CrossRef]
- Jayasooriya, U.; Peck, J.N.; Barclay, J.E.; Hardy, S.M.; Chumakov, A.I.; Evans, D.J.; Pickett, C.; Oganesyan, V.S. Nuclear inelastic scattering spectroscopy of tris(acetylacetonate)iron(III); A vibrational probe via the iron atom. Chem. Phys. Lett. 2011, 518, 119–123. [Google Scholar] [CrossRef]
- Yang, C.-C.; Chiu, S.-J.; Lee, K.-T.; Chien, W.-C.; Lin, C.-T.; Huang, C.-A. Study of poly(vinyl alcohol)/titanium oxide composite polymer membranes and their application on alkaline direct alcohol fuel cell. J. Power Sources 2008, 184, 44–51. [Google Scholar] [CrossRef]
- Schmid, T.; Messmer, A.; Yeo, B.S.; Zhang, W.; Zenobi, R. Towards chemical analysis of nanostructures in biofilms II: tip-enhanced Raman spectroscopy of alginates. Anal. Bioanal. Chem. 2008, 391, 1907–1916. [Google Scholar] [CrossRef] [Green Version]
- Glidewell, C. Metal Acetylacetonate Complexes: Preparation and Characterization. In Inorganic Experiments, 3rd ed.; Wiley-VCH: Weinheim, Germany, 2010; p. 117. [Google Scholar]
- Merkel, T.C. Ultrapermeable, Reverse-Selective Nanocomposite Membranes. Science 2002, 296, 519–522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matteucci, S.; Kusuma, V.A.; Sanders, D.; Swinnea, S.; Freeman, B.D. Gas transport in TiO2 nanoparticle-filled poly(1-trimethylsilyl-1-propyne). J. Membr. Sci. 2008, 307, 196–217. [Google Scholar] [CrossRef]
- Ahn, J.; Chung, W.-J.; Pinnau, I.; Song, J.; Du, N.; Robertson, G.P.; Guiver, M.D. Gas transport behavior of mixed-matrix membranes composed of silica nanoparticles in a polymer of intrinsic microporosity (PIM-1). J. Membr. Sci. 2010, 346, 280–287. [Google Scholar] [CrossRef] [Green Version]
- Kuila, S.B.; Ray, S.K. Dehydration of dioxane by pervaporation using filled blend membranes of poly (vinyl alcohol) and sodium alginate. Carbohydr. Polym. 2014, 101, 1154–1165. [Google Scholar] [CrossRef]
- Kulkarni, R.V.; Sreedhar, V.; Mutalik, S.; Setty, C.; Sa, B. Interpenetrating network hydrogel membranes of sodium alginate and poly(vinyl alcohol) for controlled release of prazosin hydrochloride through skin. Int. J. Boil. Macromol. 2010, 47, 520–527. [Google Scholar] [CrossRef]
- Sabarudin, A.; Wahid, R.; Nalle, F.C.; Shobirin, R.A.; Santjojo, D.J.D.H. Designed structure and magnetic characteristic studies of magnetic iron oxide (Fe3O4) nanoparticles coated by polyvinyl alcohol and polyvinyl alcohol-linked with glutaraldehyde. Rasayan J. Chem. 2017, 10, 1261–1270. [Google Scholar]
- Germanos, G.; Youssef, S.; Farah, W.; Lescop, B.; Rioual, S.; Abboud, M. The impact of the magnetite nanoparticles on the physicochemical and adsorption properties of the magnetic alginate beads. J. Environ. Chem. Eng. 2020, 8, 104223. [Google Scholar] [CrossRef]
- Nidhin, M.; Sreeram, K.J.; Nair, B.U. Polysaccharide films as templates in the synthesis of hematite nanostructures with special properties. Appl. Surf. Sci. 2012, 258, 5179–5184. [Google Scholar] [CrossRef]
- Bala, T.; Prasad, B.L.V.; Sastry, M.; Kahaly, M.U.; Waghmare, U.V. Interaction of Different Metal Ions with Carboxylic Acid Group: A Quantitative Study. J. Phys. Chem. A 2007, 111, 6183–6190. [Google Scholar] [CrossRef]
- Dudek, G.; Gnus, M.; Turczyn, R.; Strzelewicz, A.; Krasowska, M. Pervaporation with chitosan membranes containing iron oxide nanoparticles. Sep. Purif. Technol. 2014, 133, 8–15. [Google Scholar] [CrossRef]








© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dudek, G.; Turczyn, R.; Djurado, D. Collation Efficiency of Poly(Vinyl Alcohol) and Alginate Membranes with Iron-Based Magnetic Organic/Inorganic Fillers in Pervaporative Dehydration of Ethanol. Materials 2020, 13, 4152. https://doi.org/10.3390/ma13184152
Dudek G, Turczyn R, Djurado D. Collation Efficiency of Poly(Vinyl Alcohol) and Alginate Membranes with Iron-Based Magnetic Organic/Inorganic Fillers in Pervaporative Dehydration of Ethanol. Materials. 2020; 13(18):4152. https://doi.org/10.3390/ma13184152
Chicago/Turabian StyleDudek, Gabriela, Roman Turczyn, and David Djurado. 2020. "Collation Efficiency of Poly(Vinyl Alcohol) and Alginate Membranes with Iron-Based Magnetic Organic/Inorganic Fillers in Pervaporative Dehydration of Ethanol" Materials 13, no. 18: 4152. https://doi.org/10.3390/ma13184152