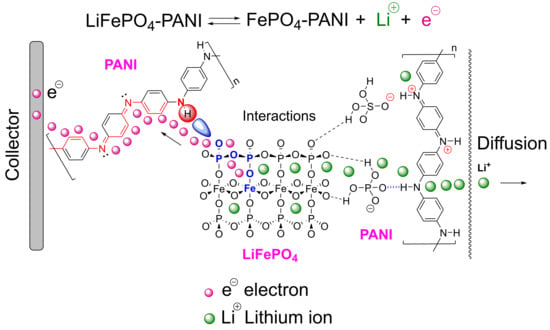
Synthesis and Characterization of LiFePO4–PANI Hybrid Material as Cathode for Lithium-Ion Batteries
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials Synthesis of PANI
2.2. Synthesis of LiFePO4–PANI
2.3. Structural Characterization
2.4. Electrode Preparation and Electrochemical Characterization
3. Results and Discussion
3.1. Synthesis of PANI and LiFePO4–PANI
3.2. PXRD Characterizations of LiFePO4, PANI and LiFePO4–PANI
3.3. Morphologies of LiFePO4, PANI and LiFePO4–PANI
3.4. FT-Infrared Spectroscopy

3.5. Thermogravimetric Analysis
3.6. Electrochemical Characterization
4. Conclusions
Prime Novelty Statement
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Padhi, A.K.; Nanjundaswamy, K.S.; Goodenough, J.B. Phospho-olivines as Positive-Electrode Materials for Rechargeable Lithium Batteries. J. Electrochem. Soc. 1997, 144, 1188–1194. [Google Scholar] [CrossRef]
- Ajpi, C.; Diaz, G.; Visbal, H.; Hirao, K. Synthesis and characterization of Cu-doped LiFePO4 with/without carbon coating for cathode of lithium-ion batteries. J. Ceram. Soc. Jpn. 2013, 121, 441–443. [Google Scholar] [CrossRef] [Green Version]
- Lin, H.B.; Zhang, Y.M.; Hua, J.N.; Wang, Y.T.; Xing, L.; Xu, M.Q.; Li, X.P.; Li, W.S. LiNi0.5Mn1.5O4 nanoparticles: Synthesis with synergistic effect of polyvinylpyrrolidone and ethylene glycol and performance as cathode of lithium ion battery. J. Power Sources 2014, 257, 37–44. [Google Scholar] [CrossRef]
- Villca, J.; Vargas, M.; Yapu, W.; Blanco, M.; Benavente, F.; Cabrera, S. Nueva ruta pirolitico/atrano para la síntesis de electrodos catódicos LiCoO2 y LiMn2O4. Revista Boliviana de Química 2014, 31, 82–85. [Google Scholar]
- Lee, M.; Hong, J.; Kim, H.; Lim, H.-D.; Cho, S.B.; Kang, K.; Park, C.B. Organic Nanohybrids for Fast and Sustainable Energy Storage. Adv. Mater. 2014, 26, 2558–2565. [Google Scholar] [CrossRef] [PubMed]
- Morales, J.; Trocoli, R.; Rodriguez-Castellon, E.; Franger, S.; Santos-Pena, J. Effect of C and Au additives produced by simple coaters on the surface and the electrochemical properties of nanosized LiFePO4. J. Electroanal. Chem. 2009, 631, 29–35. [Google Scholar] [CrossRef]
- Chung, S.Y.; Bloking, J.T.; Chiang, Y.M. Electronically conductive phospho-olivines as lithium storage electrodes. Nat. Mater. 2002, 1, 123–128. [Google Scholar] [CrossRef]
- Hiu, H.; Cao, Q.; Fu, L.J.; Li, C.; Wu, Y.P.; Wu, H.Q. Doping effects of zinc on LiFePO4 cathode material for lithium ion batteries. Electrochem. Commun. 2006, 8, 1553–1557. [Google Scholar]
- Prosini, P.P.; Carewska, M.; Scaccia, S.; Wisniewski, P.; Passerini, S. A new synthetic route for preparing LiFePO4 with enhanced electrochemical performance. J. Electrochem. Soc. 2002, 149, A886–A890. [Google Scholar] [CrossRef]
- Bruce, P.G.; Scrosati, B.; Tarascon, J.-M. Nanomaterials for rechargeable lithium batteries. Angew. Chem. Int. 2008, 47, 2930–2946. [Google Scholar] [CrossRef]
- Park, K.S.; Schougaard, S.B.; Goodenough, J.B. Conducting-Polymer/Iron-Redox- Couple Composite Cathodes for Lithium Secondary Batteries. Adv. Mater. 2007, 19, 848–851. [Google Scholar] [CrossRef]
- Chen, W.-M.; Huang, Y.-H.; Yuan, L.-X. Self-assembly LiFePO4/polyaniline composite cathode materials with inorganic acids as dopants for lithium-ion batteries. J. Electroanal. Chem. 2011, 660, 108–113. [Google Scholar] [CrossRef]
- Abdiryim, T.; Zhang, X.-G.; Jamal, R. Comparative studies of solid-state synthesized polyaniline doped with inorganic acids. Mater. Chem. Phys. 2005, 90, 367–372. [Google Scholar] [CrossRef]
- Sisbandini, C.; Brandell, D.; Gustafsson, T.; Nyholm, L. The Mechanism of Capacity Enhancement in LiFePO4 Cathodes through Polyetheramine Coating. J. Electrochem. Soc. 2009, 156, A720–A725. [Google Scholar] [CrossRef]
- Ayad, M.; Zaghlol, S. Nanostructured crosslinked polyaniline with high surface area: Synthesis, characterization and adsorption for organic dye. Chem. Eng. J. 2012, 204–206, 79–86. [Google Scholar] [CrossRef]
- Chatterjee, M.J.; Ghosh, A.; Mondal, A.; Banerjee, D. Polyaniline–single walled carbon nanotube composite—A photocatalyst to degrade rose bengal and methyl orange dyes under visible-light illumination. RSC Adv. 2017, 7, 36403–36415. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.X.; Virji, S.; Weiller, B.H.; Kaner, R.B. Polyaniline nanofibers: Facile synthesis and chemical sensors. J. Am. Chem. Soc. 2003, 125, 314. [Google Scholar] [CrossRef]
- Zhang, Z.; Wan, M.; Wei, Y. Highly Crystalline Polyaniline Nanostructures Doped with Dicarboxylic Acids. Adv. Funct. Mater. 2006, 16, 1100–1104. [Google Scholar] [CrossRef]
- Fonseca, C.P.; Neves, S. The usefulness of a LiMn2O4 composite as an active cathode material in lithium batteries. J. Power Sources 2004, 135, 249. [Google Scholar] [CrossRef]
- Wang, Y.G.; Wu, W.; Cheng, L.; He, P.; Wang, C.X.Y.; Xia, Y. A polyaniline-intercalated layered manganese oxide nanocomposite prepared by an inorganic/organic interface reaction and its high electrochemical performance for Li storage. Adv. Mater. 2008, 20, 2166. [Google Scholar] [CrossRef]
- Kang, S.-G.; Man Kim, K.; Park, N.-G.; Sun Ryu, K.; Chang, S.-H. Factors affecting the electrochemical performance of organic/V2O5 hybrid cathode materials. J. Power Sources 2004, 133, 263–267. [Google Scholar] [CrossRef]
- Karthikeyan, K.; Amaresh, S.; Aravindan, V.; Kim, W.S.; Namd, K.W.; Yang, X.Q.; Lee, Y.S. Li(Mn1/3Ni1/3Fe1/3)O2 Polyaniline hybrids as cathode active material with ultra-fast chargeedischarge capability for lithium batteries. J. Power Sources 2013, 232, 240–245. [Google Scholar] [CrossRef]
- Novák, P.; Müller, K.; Santhanam, K.S.V.; Haas, O. Electrochemically active polymers for rechargeable batteries. Chem. Rev. 1997, 97, 207. [Google Scholar]
- Chen, S.A.; Lin, L.C. Polyaniline Doped by the New Class of Dopant, Ionic Salt: Structure and Properties. Macromolecules 1995, 28, 1239. [Google Scholar] [CrossRef]
- Zhang, Z.; Wan, M. Nanostructures of polyaniline composites containing nano-magnet. Synth. Mater. 2003, 132, 205–212. [Google Scholar] [CrossRef]
- Kim, B.K.; Kim, Y.H.; Won, K.; Chang, H.; Choi, Y.; Kong, K.; Rhyu, B.W.; Kim, J.J.; Lee, J.O. Electrical properties of polyaniline nanofibre synthesized with biocatalyst. Nanotechnology 2005, 16, 1177–1181. [Google Scholar] [CrossRef]
- Ding, H.; Long, Y.; Shen, J.; Wan, M.; Wei, Y. Fe2(SO4)3 as a binary oxidant and dopant to thin polyaniline nanowires with high conductivity. J. Phys. Chem. B 2010, 114, 115–119. [Google Scholar] [CrossRef]
- Zhang, Z.; Wei, Z.; Wan, M. Nanostructures of polyaniline doped with inorganic acids. Macromolecules 2002, 35, 5937–5942. [Google Scholar] [CrossRef]
- Sanchez, C.; Rozes, L.; Ribot, F.; Laberty-Robert, C.; Grosso, D.; Sassoye, C.; Boissiere, C.; Nicole, L. Chimie douce: A land of opportunities for the designed construction of functional inorganic and hybrid organic-inorganic nanomaterials. Comptes Rendus Chim. 2010, 13, 3–39. [Google Scholar] [CrossRef] [Green Version]
- Mostafaei, A.; Zolriasatein, A. Synthesis and characterization of conducting polyaniline nanocomposites containing ZnO nanorods. Prog. Nat. Sci. Mater. Int. 2012, 22, 273–280. [Google Scholar] [CrossRef] [Green Version]
- Pouget, J.P.; Jozefowicz, M.E.; Epstein, A.J.; Tang, A.G. X-ray structure of Polyaniline. Macromolecules 1991, 24, 779–789. [Google Scholar] [CrossRef]
- Paques-Ledent, M.T.; Tarte, P. Vibrational studies of olivine-typecompounds II orthophosphates, arsenates and vanadates AIBIIXVO4. Spectrochim. Acta Part A Mol. Spectrosc. 1974, 30, 673–689. [Google Scholar] [CrossRef]
- Li, W.; Hwanga, J.; Chang, W.; Setiadi, H.; Chung, K.Y.; Kim, J. Ultrathin and uniform carbon-layer-coated hierarchically porous LiFePO4 microspheres and their electrochemical performance. J. Supercrit. Fluids 2016, 116, 164–171. [Google Scholar] [CrossRef]
- Salah, A.A.; Jozwiak, P.; Zaghib, K.; Garbarczyk, J.; Gendron, F.; Mauger, A.; Julien, C. FTIR features of lithium-iron phosphates as electrode materials for rechargeable lithium batteries. Spectrochim. Acta Part. A 2006, 65, 1007–1013. [Google Scholar] [CrossRef]
- Neoh, K.G.; Kang, E.T.; Tan, K.L. Evolution of polyaniline structure during synthesis. Polymer 1993, 34, 3921–3928. [Google Scholar] [CrossRef]
- Nagaraja, M.; Mahesh, H.M.; Manjanna, J.; Rajanna, K.; Kurian, M.Z.; Lokesh, S.V. Effect of Multiwall Carbon Nanotubes on Electrical and Structural Properties of Polyaniline. J. Electron. Mater. 2012, 41, 1882–1885. [Google Scholar] [CrossRef]
- Hatchett, D.W.; Josowicz, M.; Janata, J. Acid Doping of Polyaniline: Spectroscopic and Electrochemical Studies. J. Phys. Chem. B 1999, 103, 10992–10998. [Google Scholar] [CrossRef]
- Tian, B.B.; Ning, G.-H.; Gao, Q.; Tan, L.M.; Tang, W.; Chen, Z.; Su, C.; Loh, K.P. Crystal Engineering of Naphthalenediimide-Based Metal-Organic Frameworks: Structure-Dependent Lithium Storage. ACS Appl. Mater. Interfaces 2016, 8, 31067–31075. [Google Scholar] [CrossRef]
- Shi, Y.; Shen, M.; Xu, S.; Qiu, X.; Jiang, L.; Qiang, Y.-H.; Zhuang, Q.C.; Sun, S. Electrochemical Impedance Spectroscopic Study of the Electronic and Ionic Transport Properties of NiF2/C Composites. Int. J. Electrochem. 2011, 6, 3399–3415. [Google Scholar]
- Fagundesa, W.S.; Xaviera, F.F.S.; Santanaa, L.K.; Azevedoa, M.E.; Canobrea, S.C.; Amarala, F.A. PAni-coated LiFePO4 Synthesized by a Low Temperature Solvothermal Method. Mater. Res. 2019, 22, e20180566. [Google Scholar] [CrossRef]
- Gong, W.; Chen, Y.; Sun, B.; Chen, H. Electrochemical performance of LiFePO4 coated by nano-polyaniline coater for lithium-ion batteries. Adv. Mat. Res. 2010, 146, 551–555. [Google Scholar]
- Bai, Y.-M.; Qiu, P.; Wen, Z.-L.; Han, S.-C. Synthesis and electrochemical properties of LiFePO4/PANI composites. Chin. J. Nonferr. Met. 2010, 28, 8. [Google Scholar]
- Gong, C.; Deng, F.; Tsui, C.-P.; Xue, Z.; Ye, Y.S.; Tang, C.-Y.; Zhou, X.; Xie, X. PANI–PEG copolymer modified LiFePO4 as a cathode material for high-performance lithium ion batteries. J. Mater. Chem. A 2014, 2, 19315. [Google Scholar] [CrossRef]
- Gong, Q.; He, Y.-S.; Yang, Y.; Liao, X.-Z.; Ma, Z.-F. Synthesis and electrochemical characterization of LiFePO4/C-polypyrrole composite prepared by simple chemical vapor deposition method. J. Solid State Electrochem. 2012, 16, 1383–1388. [Google Scholar] [CrossRef] [Green Version]






















| Material | R1 (Ohm) | Rct (Ohm) |
|---|---|---|
| LiFePO4 as assembled (OCV) | 14.13 | 178 |
| LiFePO4–PANI as assembled (OCV) | 3.58 | 61.1 |
| PANI as assembled (OCV) | 4.12 | 238 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ajpi, C.; Leiva, N.; Vargas, M.; Lundblad, A.; Lindbergh, G.; Cabrera, S. Synthesis and Characterization of LiFePO4–PANI Hybrid Material as Cathode for Lithium-Ion Batteries. Materials 2020, 13, 2834. https://doi.org/10.3390/ma13122834
Ajpi C, Leiva N, Vargas M, Lundblad A, Lindbergh G, Cabrera S. Synthesis and Characterization of LiFePO4–PANI Hybrid Material as Cathode for Lithium-Ion Batteries. Materials. 2020; 13(12):2834. https://doi.org/10.3390/ma13122834
Chicago/Turabian StyleAjpi, Cesario, Naviana Leiva, Max Vargas, Anders Lundblad, Göran Lindbergh, and Saul Cabrera. 2020. "Synthesis and Characterization of LiFePO4–PANI Hybrid Material as Cathode for Lithium-Ion Batteries" Materials 13, no. 12: 2834. https://doi.org/10.3390/ma13122834