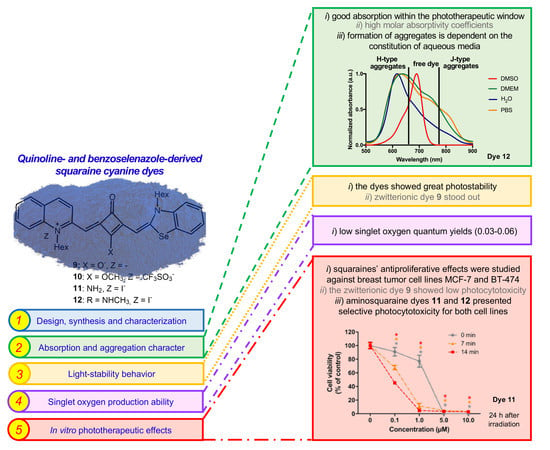
Quinoline- and Benzoselenazole-Derived Unsymmetrical Squaraine Cyanine Dyes: Design, Synthesis, Photophysicochemical Features and Light-Triggerable Antiproliferative Effects against Breast Cancer Cell Lines
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemistry
2.1.1. Synthesis of 3-Butoxy-4-[(1-hexylquinolin-2(1H)-ylidene)methyl]-cyclobut-3-en-1,2-dione (5)
2.1.2. Synthesis of 3-[(1-Hexylquinolin-2(1H)-ylidene)methyl]-4-hydroxycyclobut-3-ene-1,3-dione (6)
2.1.3. Synthesis of 2-[(3-Hexylbenzoselenazol-2(3H)-ylidene)methyl]-4-[(1-hexylquinolin-1-ium-2-yl)methylidene]-3-oxocyclobut-1-en-1-olate (9)
2.1.4. Synthesis of 1-Hexyl-2-[3-(3-hexylbenzoselenazol-2(3H)-ylidenemethyl)-2-methoxy-4-oxocyclobut-2-enylidenemethyl]quinolin-1-ium trifluoromethanesulfonate (10)
2.1.5. Synthesis of 2-[2-Amino-3-(3-hexylbenzoselenazol-2(3H)-ylidenemethyl)-4-oxocyclobut-2-enylidenemethyl]-1-hexylquinolin-1-ium Iodide (11)
2.1.6. Synthesis of 1-Hexyl-2-[3-(3-hexylbenzoselenazol-2(3H)-ylidenemethyl)-2-methylamino-4-oxocyclobut-2-enylidenemethyl]-quinolin-1-ium Iodide (12)
2.2. Photostability Assessment
2.3. Dyes’ Singlet Oxygen Quantum Yields Determination
2.4. In Vitro Photobiological Assays
3. Results
3.1. Synthesis and Photophysicochemical Characterization
3.1.1. Dyes’ Synthesis Strategy
3.1.2. Nuclear Magnetic Resonance Particularities
3.1.3. Absorption and Aggregation Behavior
3.1.4. Photostable Character
3.1.5. Singlet Oxygen Formation Ability
3.2. In Vitro Photoantiproliferative Effects
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Torre, L.A.; Islami, F.; Siegel, R.L.; Ward, E.M.; Jemal, A. Global cancer in women: Burden and trends. Cancer Epidemiol. Biomark. Prev. 2017, 26, 444–457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Global Burden of Disease Cancer Collaboration. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived with Disability, and Disability-Adjusted Life-Years for 29 Cancer Groups, 1990 to 2016: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol. 2018, 4, 1553–1568. [Google Scholar] [CrossRef] [PubMed]
- Möller, S.; Mucci, L.A.; Harris, J.R.; Scheike, T.; Holst, K.; Halekoh, U.; Adami, H.-O.; Czene, K.; Christensen, K.; Holm, N.V.; et al. The Heritability of Breast Cancer among Women in the Nordic Twin Study of Cancer. Cancer Epidemiol. Biomark. Prev. 2016, 25, 145–150. [Google Scholar] [CrossRef] [Green Version]
- Stratton, M.R.; Rahman, N. The emerging landscape of breast cancer susceptibility. Nat. Genet. 2007, 40, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Lamberti, M.J.; Vittar, N.B.R.; Rivarola, V.A. Breast cancer as photodynamic therapy target: Enhanced therapeutic efficiency by overview of tumor complexity. World J. Clin. Oncol. 2014, 5, 901–907. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Y.-S.; Zhao, Z.; Yang, Z.-N.; Xu, F.; Lu, H.-J.; Zhu, Z.-Y.; Shi, W.; Jiang, J.; Yao, P.-P.; Zhu, H.-P. Risk Factors and Preventions of Breast Cancer. Int. J. Boil. Sci. 2017, 13, 1387–1397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akram, M.; Iqbal, M.; Daniyal, M.; Khan, A.U. Awareness and current knowledge of breast cancer. Biol. Res. 2017, 50, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdel-Kader, M.H. Chapter 1. The Journey of PDT throughout History: PDT from Pharos to Present. In Photodynamic Medicine: From Bench to Clinic; Royal Society of Chemistry (RCS): London, UK, 2016; pp. 1–21. [Google Scholar]
- Dolmans, D.E.; Fukumura, D.; Jain, R.K. Photodynamic therapy for cancer. Nat. Rev. Cancer 2003, 3, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Allison, R.R.; Moghissi, K. Oncologic photodynamic therapy: Clinical strategies that modulate mechanisms of action. Photodiagn. Photodyn. Ther. 2013, 10, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.K. Photodynamic therapy: Current role in the treatment of chorioretinal conditions. Eye 2016, 30, 202–210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davis, R.K. Photodynamic therapy in otolaryngology-head and neck surgery. Otolaryngol. Clin. N. Am. 1990, 23, 107–119. [Google Scholar]
- Copper, M.P.; Tan, I.B.; Oppelaar, H.; Ruevekamp, M.C.; Stewart, F.A. Meta-tetra(hydroxyphenyl)chlorin Photodynamic Therapy in Early-Stage Squamous Cell Carcinoma of the Head and Neck. Arch. Otolaryngol. Head Neck Surg. 2003, 129, 709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quirk, B.J.; Brandal, G.; Donlon, S.; Vera, J.C.; Mang, T.S.; Foy, A.; Lew, S.M.; Girotti, A.W.; Jogal, S.; LaViolette, P.S.; et al. Photodynamic therapy (PDT) for malignant brain tumors—Where do we stand? Photodiagn. Photodyn. Ther. 2015, 12, 530–544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Minniti, G.; Filippi, A.R.; Osti, M.F.; Ricardi, U. Radiation therapy for older patients with brain tumors. Radiat. Oncol. 2017, 12, 101. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.H.; Choi, M.G.; Hasan, T. Application of photodynamic therapy in gastrointestinal disorders: An outdated or re-emerging technique? Korean J. Intern. Med. 2017, 32, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Berger, A.P.; Steiner, H.; Stenzl, A.; Akkad, T.; Bartsch, G.; Holtl, L. Photodynamic therapy with intravesical instillation of 5-aminolevulinic acid for patients with recurrent superficial bladder cancer: A single-center study. Urology 2003, 61, 338–341. [Google Scholar] [CrossRef]
- Agostinis, P.; Berg, K.; Cengel, K.A.; Foster, T.H.; Girotti, A.W.; Gollnick, S.O.; Hahn, S.M.; Hamblin, M.R.; Juzeniene, A.; Kessel, D.; et al. Photodynamic therapy of cancer: An update. CA Cancer J. Clin. 2011, 61, 250–281. [Google Scholar] [CrossRef] [PubMed]
- Buytaert, E.; Dewaele, M.; Agostinis, P. Molecular effectors of multiple cell death pathways initiated by photodynamic therapy. Biochim. Biophys. Acta Bioenerg. 2007, 1776, 86–107. [Google Scholar] [CrossRef] [PubMed]
- Allison, R.R.; Moghissi, K. Photodynamic Therapy (PDT): PDT Mechanisms. Clin. Endosc. 2013, 46, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Abrahamse, H.; Hamblin, M.R. New photosensitizers for photodynamic therapy. Biochem. J. 2016, 473, 347–364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dougherty, T.J.; Kaufman, J.E.; Goldfarb, A.; Weishaupt, K.R.; Boyle, D.; Mittleman, A. Photoradiation therapy for the treatment of malignant tumors. Cancer Res. 1978, 38, 2628–2635. [Google Scholar] [PubMed]
- Bäumler, W. Chapter 6. Light sources for photodynamic therapy and fluorescence diagnosis in dermatology. In Comprehensive Series in Photosciences; Elsevier BV: Aalborg, Denmark, 2001; pp. 83–100. [Google Scholar]
- Brancaleon, L.; Moseley, H. Laser and Non-laser Light Sources for Photodynamic Therapy. Lasers Med. Sci. 2002, 17, 173–186. [Google Scholar] [CrossRef] [PubMed]
- Lima, E.; Ferreira, O.; Gomes, V.S.; Santos, A.O.; Boto, R.E.; Fernandes, J.R.; Almeida, P.; Silvestre, S.M.; Reis, L.V. Synthesis and in vitro evaluation of the antitumoral phototherapeutic potential of squaraine cyanine dyes derived from indolenine. Dye Pigm. 2019, 167, 98–108. [Google Scholar] [CrossRef]
- Wang, X.; Hu, J.; Wang, P.; Zhang, S.; Liu, Y.; Xiong, W.; Liu, Q. Analysis of the In Vivo and In Vitro Effects of Photodynamic Therapy on Breast Cancer by Using a Sensitizer, Sinoporphyrin Sodium. Theranostics 2015, 5, 772–786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dimofte, A.; Zhu, T.C.; Hahn, S.; Lustig, R.A. In vivo light dosimetry for motexafin lutetium-mediated PDT of recurrent breast cancer. Lasers Surg. Med. 2002, 31, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.M.; El-Sheikh, S.; Malhotra, A.; Mosse, C.A.; Parker, S.; Williams, N.R.; MacRobert, A.J.; Hamoudi, R.; Bown, S.G.; Keshtgar, M.R. Photodynamic Therapy in Primary Breast Cancer. J. Clin. Med. 2020, 9, 483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Comşa, Ş.; Cimpean, A.M.; Raica, M. The Story of MCF-7 Breast Cancer Cell Line: 40 years of Experience in Research. Anticancer Res. 2015, 35, 3147–3154. [Google Scholar] [PubMed]
- Bartlett, J.; Parelukar, W. Breast cancers are rare diseases-and must be treated as such. NPJ Breast Cancer 2017, 3, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kou, J.; Dou, D.; Yang, L. Porphyrin photosensitizers in photodynamic therapy and its applications. Oncotarget 2017, 8, 81591–81603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Issa, M.C.A.; Manela-Azulay, M. Terapia fotodinâmica: Revisão da literatura e documentação iconográfica. An. Bras. Dermatol. 2010, 85, 501–511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gomes, A.; Fernandes, E.; Lima, J.L.F.C. Fluorescence probes used for detection of reactive oxygen species. J. Biochem. Biophys. Methods 2005, 65, 45–80. [Google Scholar] [CrossRef] [PubMed]
- Silva, E.F.F.; Serpa, C.; Dąbrowski, J.M.; Monteiro, C.J.P.; Formosinho, S.J.; Stochel, G.; Urbanska, K.; Simoes, S.; Pereira, M.M.; Arnaut, L.G. Mechanisms of Singlet-Oxygen and Superoxide-Ion Generation by Porphyrins and Bacteriochlorins and their Implications in Photodynamic Therapy. Chem. Eur. J. 2010, 16, 9273–9286. [Google Scholar] [CrossRef] [PubMed]
- Treibs, A.; Jacob, K. Cyclotrimethine Dyes Derived from Squaric Acid. Angew. Chem. Int. Ed. 1965, 4, 694. [Google Scholar] [CrossRef]
- Avirah, R.R.; Jayaram, D.T.; Adarsh, N.; Ramaiah, D. Squaraine dyes in PDT: From basic design to in vivo demonstration. Org. Biomol. Chem. 2012, 10, 911–920. [Google Scholar] [CrossRef] [PubMed]
- Friães, S.; Silva, A.M.; Boto, R.E.; Ferreira, D.; Fernandes, J.R.; Souto, E.B.; Almeida, P.; Ferreira, L.F.V.; Reis, L.V. Synthesis, spectroscopic characterization and biological evaluation of unsymmetrical aminosquarylium cyanine dyes. Bioorg. Med. Chem. 2017, 25, 3803–3814. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Terpetschnig, E.; Szmacinski, H.; Lakowicz, J.R. An investigation of squaraines as a new class of fluorophores with long-wavelength excitation and emission. J. Fluoresc. 1993, 3, 153–155. [Google Scholar] [CrossRef] [PubMed]
- Reis, L.V.; Serrano, J.P.; Almeida, P.; Santos, P.F. New Synthetic Approach to Aminosquarylium Cyanine Dyes. Synlett 2002, 1617–1620. [Google Scholar] [CrossRef]
- Santos, P.F.; Reis, L.V.; Almeida, P.; Oliveira, A.S.; Ferreira, L.F.V. Singlet oxygen generation ability of squarylium cyanine dyes. J. Photochem. Photobiol. Chem. 2003, 160, 159–161. [Google Scholar] [CrossRef]
- Lima, E.; Ferreira, O.; Silva, J.F.; Santos, A.O.; Boto, R.E.; Fernandes, J.R.; Almeida, P.; Silvestre, S.M.; Reis, L.V. Photodynamic activity of indolenine-based aminosquaraine cyanine dyes: Synthesis and in vitro photobiological evaluation. Dye Pigm. 2020, 174, 108024. [Google Scholar] [CrossRef]
- Reis, L.V.; Serrano, J.; Almeida, P.; Santos, P.F. The synthesis and characterization of novel, aza-substituted squarylium cyanine dyes. Dye Pigm. 2009, 81, 197–202. [Google Scholar] [CrossRef]
- Volkova, K.D.; Kovalska, V.B.; Losytskyy, M.Y.; Reis, L.V.; Santos, P.F.; Almeida, P.; Lynch, D.E.; Yarmoluk, S.M. Aza-substituted squaraines for the fluorescent detection of albumins. Dye Pigm. 2011, 90, 41–47. [Google Scholar] [CrossRef]
- Babu, P.S.S.; Manu, P.M.; Dhanya, T.J.; Tapas, P.; Meera, R.N.; Surendran, A.; Aneesh, K.A.; Vadakkancheril, S.J.; Ramaiah, D.; Nair, S.A.; et al. Bis (3,5-diiodo-2,4,6-trihydroxyphenyl) squaraine photodynamic therapy disrupts redox homeostasis and induce mitochondria-mediated apoptosis in human breast cancer cells. Sci. Rep. 2017, 7, 42126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramaiah, D.; Joy, A.; Chandrasekhar, N.; Eldho, N.V.; Das, S.; George, M.V. Halogenated Squaraine Dyes as Potential Photochemotherapeutic Agents. Synthesis and Study of Photophysical Properties and Quantum Efficiencies of Singlet Oxygen Generation. Photochem. Photobiol. 1997, 65, 783–790. [Google Scholar] [CrossRef]
- Amarego, W.L.F.; Perrin, D.D. Purification of Laboratory Chemicals, 4th ed.; Butterworth-Heinemann: Oxford, UK, 1996; ISBN 0-7506-3761-7. [Google Scholar]
- Pardal, A.; Ramos, S.S.; Santos, P.F.; Reis, L.V.; Almeida, P. Synthesis and Spectroscopic Characterisation of N-Alkyl Quaternary Ammonium Salts Typical Precursors of Cyanines. Molecules 2002, 7, 320–330. [Google Scholar] [CrossRef]
- Friães, S.; Lima, E.; Boto, R.E.; Ferreira, D.; Fernandes, J.R.; Ferreira, L.F.V.; Silva, A.M.; Reis, L.V. Photophysicochemical Properties and In Vitro Phototherapeutic Effects of Iodoquinoline—And Benzothiazole-Derived Unsymmetrical Squaraine Cyanine Dyes. Appl. Sci. 2019, 9, 5414. [Google Scholar] [CrossRef] [Green Version]
- Souto, E.B.; Doktorovova, S.; Campos, J.R.; Martins-Lopes, P.; Silva, A.M. Surface-tailored anti-HER2/neu-solid lipid nanoparticles for site-specific targeting MCF-7 and BT-474 breast cancer cells. Eur. J. Pharm. Sci. 2019, 128, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Andreani, T.; Kiill, C.P.; De Souza, A.L.R.; Fangueiro, J.; Fernandes, L.S.G.; Doktorovova, S.; Santos, D.; Garcia, M.L.; Gremião, M.P.D.; Souto, E.B.; et al. Surface engineering of silica nanoparticles for oral insulin delivery: Characterization and cell toxicity studies. Colloids Surf. B Biointerfaces 2014, 123, 916–923. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, A.M.; Martins-Gomes, C.; Coutinho, T.E.; Fangueiro, J.F.; Sánchez-López, E.; Pashirova, T.N.; Andreani, T.; Souto, E.B. Soft Cationic Nanoparticles for Drug Delivery: Production and Cytotoxicity of Solid Lipid Nanoparticles (SLNs). Appl. Sci. 2019, 9, 4438. [Google Scholar] [CrossRef] [Green Version]
- Dąbrowski, J.M.; Arnaut, L.G. Photodynamic therapy (PDT) of cancer: From local to systemic treatment. Photochem. Photobiol. Sci. 2015, 14, 1765–1780. [Google Scholar] [CrossRef] [PubMed]
- Diwu, Z.; Lown, J.W. Phototherapeutic potential of alternative photosensitizers to porphyrins. Pharmacol. Ther. 1994, 63, 1–35. [Google Scholar] [CrossRef]
- Deng, H.; Li, T.; Xie, J.; Huang, N.; Gu, Y.; Zhao, J. Synthesis and bio-evaluation of novel hypocrellin derivatives: Potential photosensitizers for photodynamic therapy of age-related macular degeneration. Dye Pigm. 2013, 99, 930–939. [Google Scholar] [CrossRef]
- Dąbrowski, J.M.; Pucelik, B.; Regiel-Futyra, A.; Brindell, M.; Mazuryk, O.; Kyzioł, A.; Stochel, G.; Macyk, W.; Arnaut, L.G. Engineering of relevant photodynamic processes through structural modifications of metallotetrapyrrolic photosensitizers. Coord. Chem. Rev. 2016, 325, 67–101. [Google Scholar] [CrossRef]
- Dempsey, J.; Zhang, Q.-W.; Oliver, A.G.; Smith, B.D. New tetralactam hosts for squaraine dyes. Org. Biomol. Chem. 2018, 16, 8976–8983. [Google Scholar] [CrossRef] [PubMed]
- Paternò, G.M.; Barbero, N.; Galliano, S.; Barolo, C.; Lanzani, G.; Scotognella, F.; Borrelli, R. Excited state photophysics of squaraine dyes for photovoltaic applications: An alternative deactivation scenario. J. Mater. Chem. C 2018, 6, 2778–2785. [Google Scholar] [CrossRef] [Green Version]
- Pascal, S.; Haefele, A.; Monnereau, C.; Charaf-Eddin, A.; Jacquemin, D.; Le Guennic, B.; Andraud, C.; Maury, O. Expanding the Polymethine Paradigm: Evidence for the Contribution of a Bis-Dipolar Electronic Structure. J. Phys. Chem. A 2014, 118, 4038–4047. [Google Scholar] [CrossRef] [PubMed]
- Hassan, G.F.; Saad, N.E.H.; Hmadeh, M.; Karam, P. Enhancing porphyrin photostability when locked in metal—Organic frameworks. Dalton Trans. 2018, 47, 15765–15771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Merchan, M.; Ouk, T.-S.; Kubát, P.; Lang, K.; Coelho, C.; Verney, V.; Commereuc, S.; Leroux, F.; Sol, V.; Taviot-Gueho, C. Photostability and photobactericidal properties of porphyrin-layered double hydroxide-polyurethane composite films. J. Mater. Chem. B 2013, 1, 2139–2146. [Google Scholar] [CrossRef] [PubMed]
- Dąbrowski, J.M.; Arnaut, L.G.; Pereira, M.; Monteiro, C.; Urbanska, K.; Simoes, S.; Stochel, G. New Halogenated Water-Soluble Chlorin and Bacteriochlorin as Photostable PDT Sensitizers: Synthesis, Spectroscopy, Photophysics, and in vitro Photosensitizing Efficacy. ChemMedChem 2010, 5, 1770–1780. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, J.; Menezes, P.F.C.; Sibata, C.H.; Allison, R.R.; Zucoloto, S.; E Silva, O.C.; Bagnato, V.S.; Silva, O.C.E., Jr.; Bagnato, V.S. Can efficiency of the photosensitizer be predicted by its photostability in solution? Laser Phys. 2009, 19, 1932–1938. [Google Scholar] [CrossRef]
- Menezes, P.F.C.; Imasato, H.; Ferreira-Strixino, J.; Bagnato, V.S.; Sibata, C.H.; Perussi, J.R. Aggregation susceptibility on phototransformation of hematoporphyrin derivatives. Laser Phys. Lett. 2008, 5, 227–235. [Google Scholar] [CrossRef]
- Ahmad, I.; Ahmed, S.; Anwar, Z.; Sheraz, M.A.; Sikorski, M. Photostability and Photostabilization of Drugs and Drug Products. Int. J. Photoenergy 2016, 2016, 8135608. [Google Scholar] [CrossRef] [Green Version]
- Welankiwar, A.; Saudagar, S.; Kumar, J.; Barabde, A. Photostability Testing of Pharmaceutical Products. Int. Res. J. Pharm. 2013, 2, 11–15. [Google Scholar] [CrossRef]
- Krasnovsky, A.J. Singlet oxygen and primary mechanisms of photodynamic therapy and photodynamic diseases. In Photodynamic Therapy at the Cellular Level; Research Signpost: Thiruvananthapuram, India, 2007; pp. 17–62. ISBN 978-81-308-0174-2. [Google Scholar]
- DeRosa, M.; Crutchley, R.J. Photosensitized singlet oxygen and its applications. Coord. Chem. Rev. 2002, 233, 351–371. [Google Scholar] [CrossRef]
- Wu, W.; Shao, X.; Zhao, J.; Wu, M. Controllable Photodynamic Therapy Implemented by Regulating Singlet Oxygen Efficiency. Adv. Sci. 2017, 4, 1700113. [Google Scholar] [CrossRef] [PubMed]
- Sari, C.; Eyüpoğlu, F.C.; Değirmencioğlu, I.; Bayrak, R. Synthesis of axially disubstituted silicon phthalocyanines and investigation of photodynamic effects on HCT-116 colorectal cancer cell line. Photodiagn. Photodyn. Ther. 2018, 23, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Serpe, L.; Ellena, S.; Barbero, N.; Foglietta, F.; Prandini, F.; Gallo, M.P.; Levi, R.; Barolo, C.; Canaparo, R.; Visentin, S. Squaraines bearing halogenated moieties as anticancer photosensitizers: Synthesis, characterization and biological evaluation. Eur. J. Med. Chem. 2016, 113, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Rusche, B. The 3Rs and animal welfare—Conflict or the way forward? Altex 2003, 20, 63–76. [Google Scholar] [PubMed]
- Dai, X.; Cheng, H.; Bai, Z.; Li, J. Breast Cancer Cell Line Classification and Its Relevance with Breast Tumor Subtyping. J. Cancer 2017, 8, 3131–3141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balaji, S.A.; Udupa, N.; Chamallamudi, M.R.; Gupta, V.; Rangarajan, A. Role of the Drug Transporter ABCC3 in Breast Cancer Chemoresistance. PLoS ONE 2016, 11, e0155013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- International Standard (ISO). Biological Evaluation of Medical Devices. Part 5: Tests for In Vitro Cytotoxicity, 3rd ed.; ISO 10993 5; International Standard (ISO): Geneva, Switzerland, 2009. [Google Scholar]
- Doktorovova, S.; Souto, E.B.; Silva, A.M. Nanotoxicology applied to solid lipid nanoparticles and nanostructured lipid carriers—A systematic review of in vitro data. Eur. J. Pharm. Biopharm. 2014, 87, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Martins, T.D.; Lima, E.; Boto, R.E.; Ferreira, D.; Fernandes, J.R.; Almeida, P.; Ferreira, L.F.V.; Silva, A.M.; Reis, L.V. Red and Near-Infrared Absorbing Dicyanomethylene Squaraine Cyanine Dyes: Photophysicochemical Properties and Anti-Tumor Photosensitizing Effects. Materials 2020, 13, 2083. [Google Scholar] [CrossRef] [PubMed]
- Mandim, F.; Graça, V.C.; Calhelha, R.C.; Machado, I.L.F.; Ferreira, L.F.V.; Ferreira, I.C.F.R.; Santos, P.F. Synthesis, Photochemical and In Vitro Cytotoxic Evaluation of New Iodinated Aminosquaraines as Potential Sensitizers for Photodynamic Therapy. Molecules 2019, 24, 863. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Magalhães, A.F.; Graça, V.C.; Calhelha, R.C.; Ferreira, I.C.F.R.; Santos, P.F. Aminosquaraines as potential photodynamic agents: Synthesis and evaluation of in vitro cytotoxicity. Bioorg. Med. Chem. Lett. 2017, 27, 4467–4470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Magalhães, A.F.; Graça, V.C.; Calhelha, R.C.; Machado, I.L.F.; Ferreira, L.F.V.; Ferreira, I.C.F.R.; Santos, P.F.; Filipe, L. Synthesis, photochemical and in vitro cytotoxic evaluation of benzoselenazole-based aminosquaraines. Photochem. Photobiol. Sci. 2019, 18, 336–342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferreira, D.P.; Conceição, D.S.; Ferreira, V.R.A.; Graça, V.C.; Santos, P.F.; Ferreira, L.F.V. Photochemical properties of squarylium cyanine dyes. Photochem. Photobiol. Sci. 2013, 12, 1948. [Google Scholar] [CrossRef] [PubMed]






| Dye | 1H NMR | 13C NMR |
|---|---|---|
| C=CH | C=CH | |
| 9 | 6.00 (1H, s) | 92.85 |
| 5.77 (1H, s) | 88.91 | |
| 10 | 6.19 (1H, s) | 92.84 |
| 5.75 (1H, s) | 89.13 | |
| 11 | 6.18 (1H, s) | 93.89 |
| 5.95 (1H, s) | 89.15 | |
| 12 | 6.15 (1H, s) | 94.40 |
| 6.07 (1H, s) | 93.99 | |
| 5.92 (1H, s) | 89.86 | |
| 5.85 (1H, s) | 89.30 |
| Solvent | Dye 9 | Dye 10 | Dye 11 | Dye 12 | ||||
|---|---|---|---|---|---|---|---|---|
| λmax | log ε | λmax | log ε | λmax | log ε | λmax | log ε | |
| Organic Solvent | ||||||||
| ACN | 686 | 5.11 | 636 | 5.82 | 658 | 5.83 | 671 | 5.76 |
| ACT | 692 | 5.24 | 640 | 5.91 | 664 | 5.85 | 676 | 5.81 |
| DCM | 694 | 5.18 | 643 | 5.91 | 669 | 5.84 | 681 | 5.71 |
| DMF | 689 | 5.04 | 647 | 5.75 | 674 | 5.79 | 686 | 5.70 |
| DMSO | 700 | 5.20 | 651 | 5.82 | 679 | 5.80 | 690 | 5.75 |
| DXN | 700 | 5.15 | 655 | 5.73 | 680 | 5.74 | 689 | 5.69 |
| EtOH | 674 | 5.18 | 641 | 5.86 | 667 | 5.84 | 677 | 5.77 |
| MeOH | 690 | 5.28 | 661 | 5.86 | 684 | 5.81 | 695 | 5.76 |
| THF | 706 | 5.11 | 646 | 5.85 | 680 | 5.79 | 689 | 5.74 |
| XLN | 733 | 5.27 | 688 | 5.75 | 714 | 5.80 | 718 | 5.72 |
| Aqueous Solvent | ||||||||
| DMEM | 649 | 4.54 | – | – | 625 | 5.10 | 638 | 5.01 |
| H2O | 631 | 4.58 | – | – | 608 | 5.32 | 617 | 5.27 |
| PBS | 650 | 4.46 | – | – | 628 | 5.09 | 778 | 5.20 |
| ΦΔ | Dye 9 | Dye 11 | Dye 12 |
|---|---|---|---|
| CFM | 0.03 | 0.04 | 0.06 |
| Dye | Irradiation Time | BT-474 | MCF-7 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 h | 24 h | 1 h | 24 h | ||||||
| IC50 | r2 | IC50 | r2 | IC50 | r2 | IC50 | r2 | ||
| 9 | 0′ | 4.423 | 0.92 | 7.023 | 0.92 | 6.400 | 0.99 | 5.999 | 0.92 |
| 7′ | 5.628 | 0.71 | 1.958 | 0.79 | 6.335 | 0.96 | 6.359 | 0.99 | |
| 14′ | 6.941 | 0.96 | 3.184 | 0.95 | 6.414 | 0.97 | 3.149 | 0.88 | |
| 11 | 0′ | 1.209 | 0.99 | 0.706 | 0.99 | 4.018 | 0.98 | 1.225 | 0.98 |
| 7′ | 0.203 | 0.99 | 0.207 | 0.99 | 0.313 | 0.98 | 0.189 | 0.99 | |
| 14’ | 0.197 | 0.99 | 0.097 | 0.99 | 0.140 | 0.99 | 0.084 | 0.99 | |
| 12 | 0′ | 1.468 | 0.96 | 2.534 | 0.99 | 1.078 | 0.99 | 1.253 | 0.99 |
| 7′ | 0.402 | 0.98 | 0.242 | 0.99 | 0.147 | 0.99 | 0.074 | 0.99 | |
| 14′ | 0.386 | 0.99 | 0.199 | 0.99 | 0.104 | 0.99 | 0.042 | 0.99 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lima, E.; E. Boto, R.; Ferreira, D.; R. Fernandes, J.; Almeida, P.; F. V. Ferreira, L.; Souto, E.B.; Silva, A.M.; V. Reis, L. Quinoline- and Benzoselenazole-Derived Unsymmetrical Squaraine Cyanine Dyes: Design, Synthesis, Photophysicochemical Features and Light-Triggerable Antiproliferative Effects against Breast Cancer Cell Lines. Materials 2020, 13, 2646. https://doi.org/10.3390/ma13112646
Lima E, E. Boto R, Ferreira D, R. Fernandes J, Almeida P, F. V. Ferreira L, Souto EB, Silva AM, V. Reis L. Quinoline- and Benzoselenazole-Derived Unsymmetrical Squaraine Cyanine Dyes: Design, Synthesis, Photophysicochemical Features and Light-Triggerable Antiproliferative Effects against Breast Cancer Cell Lines. Materials. 2020; 13(11):2646. https://doi.org/10.3390/ma13112646
Chicago/Turabian StyleLima, Eurico, Renato E. Boto, Diana Ferreira, José R. Fernandes, Paulo Almeida, Luis F. V. Ferreira, Eliana B. Souto, Amélia M. Silva, and Lucinda V. Reis. 2020. "Quinoline- and Benzoselenazole-Derived Unsymmetrical Squaraine Cyanine Dyes: Design, Synthesis, Photophysicochemical Features and Light-Triggerable Antiproliferative Effects against Breast Cancer Cell Lines" Materials 13, no. 11: 2646. https://doi.org/10.3390/ma13112646