Red and Near-Infrared Absorbing Dicyanomethylene Squaraine Cyanine Dyes: Photophysicochemical Properties and Anti-Tumor Photosensitizing Effects
Abstract
:1. Introduction
2. Materials and Methods
2.1. Visible and Near-Infrared Absorption Spectra
2.2. Singlet Oxygen Formation Quantum Yields Determination
2.3. Photostability Analysis
2.4. In Vitro Phototherapeutic Potential Evaluation
2.4.1. Cell Culture
2.4.2. Alamar Blue Cell Proliferation Assay
2.4.3. Light-Emitting Diode System Specifications
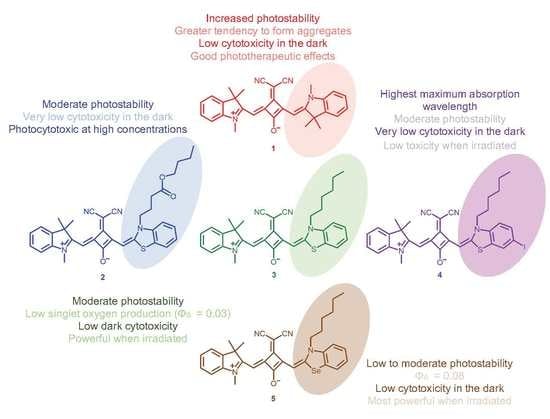
3. Results
3.1. Photophysical Parameters
3.2. Photostability Monitoring
3.3. Cell Viability Photodynamic Effects
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kwiatkowski, S.; Knap, B.; Przystupski, D.; Saczko, J.; Kędzierska, E.; Knap-Czop, K.; Kotlińska, J.; Michel, O.; Kotowski, K.; Kulbacka, J. Photodynamic therapy—Mechanisms, photosensitizers and combinations. Biomed. Pharmacother. 2018, 106, 1098–1107. [Google Scholar] [CrossRef] [PubMed]
- Buytaert, E.; Dewaele, M.; Agostinis, P. Molecular effectors of multiple cell death pathways initiated by photodynamic therapy. Biochim. Et Biophys. Acta (Bba) Rev. Cancer 2007, 1776, 86–107. [Google Scholar] [CrossRef] [PubMed]
- Van Straten, D.; Mashayekhi, V.; de Bruijn, H.; Oliveira, S.; Robinson, D. Oncologic Photodynamic Therapy: Basic Principles, Current Clinical Status and Future Directions. Cancers 2017, 9, 19. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Yu, H.; Dong, Y.; Tian, R.; Huang, G.; Boothman, D.A.; Sumer, B.D.; Gao, J. Photoactivation switch from type II to type I reactions by electron-rich micelles for improved photodynamic therapy of cancer cells under hypoxia. J. Control. Release 2011, 156, 276–280. [Google Scholar] [CrossRef] [Green Version]
- Yano, S.; Hirohara, S.; Obata, M.; Hagiya, Y.; Ogura, S.; Ikeda, A.; Kataoka, H.; Tanaka, M.; Joh, T. Current states and future views in photodynamic therapy. J. Photochem. Photobiol. C: Photochem. Rev. 2011, 12, 46–67. [Google Scholar] [CrossRef]
- Meng, Z.; Hou, W.; Zhou, H.; Zhou, L.; Chen, H.; Wu, C. Therapeutic Considerations and Conjugated Polymer-Based Photosensitizers for Photodynamic Therapy. Macromol. Rapid Commun. 2018, 39, 1700614. [Google Scholar] [CrossRef]
- Lima, E.; Ferreira, O.; Gomes, V.S.D.; Santos, A.O.; Boto, R.E.; Fernandes, J.R.; Almeida, P.; Silvestre, S.M.; Reis, L.V. Synthesis and in vitro evaluation of the antitumoral phototherapeutic potential of squaraine cyanine dyes derived from indolenine. Dye. Pigment. 2019, 167, 98–108. [Google Scholar] [CrossRef]
- Saneesh Babu, P.S.; Manu, P.M.; Dhanya, T.J.; Tapas, P.; Meera, R.N.; Surendran, A.; Aneesh, K.A.; Vadakkancheril, S.J.; Ramaiah, D.; Nair, S.A.; et al. Bis(3,5-diiodo-2,4,6-trihydroxyphenyl)squaraine photodynamic therapy disrupts redox homeostasis and induce mitochondria-mediated apoptosis in human breast cancer cells. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef] [Green Version]
- Ramaiah, D.; Joy, A.; Chandrasekhar, N.; Eldho, N.V.; Das, S.; George, M.V. Halogenated Squaraine Dyes as Potential Photochemotherapeutic Agents. Synthesis and Study of Photophysical Properties and Quantum Efficiencies of Singlet Oxygen Generation. Photochem. Photobiol. 1997, 65, 783–790. [Google Scholar] [CrossRef]
- O’Connor, A.E.; Gallagher, W.M.; Byrne, A.T. Porphyrin and Nonporphyrin Photosensitizers in Oncology: Preclinical and Clinical Advances in Photodynamic Therapy. Photochem. Photobiol. 2009, 85, 1053–1074. [Google Scholar] [CrossRef]
- Avirah, R.R.; Jayaram, D.T.; Adarsh, N.; Ramaiah, D. Squaraine dyes in PDT: From basic design to in vivo demonstration. Org. Biomol. Chem. 2012, 10, 911–920. [Google Scholar] [CrossRef] [PubMed]
- Paszko, E.; Ehrhardt, C.; Senge, M.O.; Kelleher, D.P.; Reynolds, J.V. Nanodrug applications in photodynamic therapy. Photodiagnosis Photodyn. Ther. 2011, 8, 14–29. [Google Scholar] [CrossRef] [PubMed]
- Treibs, A.; Jacob, K. Cyclotrimethine Dyes Derived from Squaric Acid. Angew. Chem. Int. Ed. Engl. 1965, 4, 694. [Google Scholar] [CrossRef]
- Chen, G.; Sasabe, H.; Igarashi, T.; Hong, Z.; Kido, J. Squaraine dyes for organic photovoltaic cells. J. Mater. Chem. A 2015, 3, 14517–14534. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, G.; Yang, L.; Wu, J.; Melkonyan, F.S.; Huang, Y.; Lu, Z.; Marks, T.J.; Facchetti, A. Novel unsymmetrical squaraine-based small molecules for organic solar cells. J. Mater. Chem. C 2018, 6, 847–854. [Google Scholar] [CrossRef]
- Martins, T.D.; Pacheco, M.L.; Boto, R.E.; Almeida, P.; Farinha, J.P.S.; Reis, L.V. Synthesis, characterization and protein-association of dicyanomethylene squaraine dyes. Dye. Pigment. 2017, 147, 120–129. [Google Scholar] [CrossRef]
- Wu, N.; Lan, J.; Yan, L.; You, J. A sensitive colorimetric and fluorescent sensor based on imidazolium-functionalized squaraines for the detection of GTP and alkaline phosphatase in aqueous solution. Chem. Commun. 2014, 50, 4438–4441. [Google Scholar] [CrossRef]
- Lee, S.; Rao, B.A.; Son, Y.-A. A highly selective fluorescent chemosensor for Hg2+ based on a squaraine–bis(rhodamine-B) derivative: Part II. Sens. Actuators B Chem. 2015, 210, 519–532. [Google Scholar] [CrossRef]
- Basheer, M.C.; Alex, S.; George Thomas, K.; Suresh, C.H.; Das, S. A squaraine-based chemosensor for Hg2+ and Pb2+. Tetrahedron 2006, 62, 605–610. [Google Scholar] [CrossRef]
- Volkova, K.D.; Kovalska, V.B.; Losytskyy, M.Y.; Reis, L.V.; Santos, P.F.; Almeida, P.; Lynch, D.E.; Yarmoluk, S.M. Aza-substituted squaraines for the fluorescent detection of albumins. Dye. Pigment. 2011, 90, 41–47. [Google Scholar] [CrossRef]
- Friães, S.; Silva, A.M.; Boto, R.E.; Ferreira, D.; Fernandes, J.R.; Souto, E.B.; Almeida, P.; Ferreira, L.F.V.; Reis, L.V. Synthesis, spectroscopic characterization and biological evaluation of unsymmetrical aminosquarylium cyanine dyes. Bioorganic Med. Chem. 2017, 25, 3803–3814. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, G.; Wang, H. Squaraine dyes: The hierarchical synthesis and its application in optical detection. J. Photochem. Photobiol. C Photochem. Rev. 2017, 31, 84–113. [Google Scholar] [CrossRef]
- Soumya, M.S.; Shafeekh, K.M.; Das, S.; Abraham, A. Symmetrical diiodinated squaraine as an efficient photosensitizer for PDT applications: Evidence from photodynamic and toxicological aspects. Chem. -Biol. Interact. 2014, 222, 44–49. [Google Scholar] [CrossRef]
- Wei, Y.; Hu, X.; Shen, L.; Jin, B.; Liu, X.; Tan, W.; Shangguan, D. Dicyanomethylene Substituted Benzothiazole Squaraines: The Efficiency of Photodynamic Therapy In Vitro and In Vivo. EBioMedicine 2017, 23, 25–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tatarets, A.; Fedyunyaeva, I.; Terpetschnig, E.; Patsenker, L. Synthesis of novel squaraine dyes and their intermediates. Dye. Pigment. 2005, 64, 125–134. [Google Scholar] [CrossRef]
- Wu, J.; Yang, D.; Wang, Q.; Yang, L.; Sasabe, H.; Sano, T.; Kido, J.; Lu, Z.; Huang, Y. Central dicyanomethylene-substituted unsymmetrical squaraines and their application in organic solar cells. J. Mater. Chem. A 2018, 6, 5797–5806. [Google Scholar] [CrossRef]
- Jin, B.; Zhang, X.; Zheng, W.; Liu, X.; Zhou, J.; Zhang, N.; Wang, F.; Shangguan, D. Dicyanomethylene-Functionalized Squaraine as a Highly Selective Probe for Parallel G-Quadruplexes. Anal. Chem. 2014, 86, 7063–7070. [Google Scholar] [CrossRef]
- Szaciłowski, K.; Macyk, W.; Drzewiecka-Matuszek, A.; Brindell, M.; Stochel, G. Bioinorganic Photochemistry: Frontiers and Mechanisms. Chem. Rev. 2005, 105, 2647–2694. [Google Scholar] [CrossRef]
- Pandey, R.K.; Constantine, S.; Tsuchida, T.; Zheng, G.; Medforth, C.J.; Aoudia, M.; Kozyrev, A.N.; Rodgers, M.A.J.; Kato, H.; Smith, K.M.; et al. Synthesis, Photophysical Properties, in Vivo Photosensitizing Efficacy, and Human Serum Albumin Binding Properties of Some Novel Bacteriochlorins. J. Med. Chem. 1997, 40, 2770–2779. [Google Scholar] [CrossRef]
- Barbero, N.; Visentin, S.; Viscardi, G. The different kinetic behavior of two potential photosensitizers for PDT. J. Photochem. Photobiol. A Chem. 2015, 299, 38–43. [Google Scholar] [CrossRef]
- Lima, E.; Ferreira, O.; Silva, J.F.; Santos, A.O.; Boto, R.E.; Fernandes, J.R.; Almeida, P.; Silvestre, S.M.; Reis, L.V. Photodynamic activity of indolenine-based aminosquaraine cyanine dyes: Synthesis and in vitro photobiological evaluation. Dye. Pigment. 2020, 174, 108024. [Google Scholar] [CrossRef]
- Santos, P.F.; Reis, L.V.; Almeida, P.; Oliveira, A.S.; Vieira Ferreira, L.F. Singlet oxygen generation ability of squarylium cyanine dyes. J. Photochem. Photobiol. A: Chem. 2003, 160, 159–161. [Google Scholar] [CrossRef]
- Boscencu, R.; Socoteanu, R.P.; Manda, G.; Radulea, N.; Anastasescu, M.; Gama, A.; Machado, I.F.; Ferreira, L.F.V. New A3B porphyrins as potential candidates for theranostic. Synthesis and photochemical behaviour. Dye. Pigment. 2019, 160, 410–417. [Google Scholar] [CrossRef]
- Severino, P.; Andreani, T.; Jäger, A.; Chaud, M.V.; Santana, M.H.A.; Silva, A.M.; Souto, E.B. Solid lipid nanoparticles for hydrophilic biotech drugs: Optimization and cell viability studies (Caco-2 & HEPG-2 cell lines). Eur. J. Med. Chem. 2014, 81, 28–34. [Google Scholar] [CrossRef]
- Andreani, T.; Kiill, C.P.; de Souza, A.L.R.; Fangueiro, J.F.; Fernandes, L.; Doktorovová, S.; Santos, D.L.; Garcia, M.L.; Gremião, M.P.D.; Souto, E.B.; et al. Surface engineering of silica nanoparticles for oral insulin delivery: Characterization and cell toxicity studies. Colloids Surf. B Biointerfaces 2014, 123, 916–923. [Google Scholar] [CrossRef] [Green Version]
- Zenkevich, E.; Sagun, E.; Knyukshto, V.; Shulga, A.; Mironov, A.; Efremova, O.; Bonnett, R.; Songca, S.P.; Kassem, M. Photophysical and photochemical properties of potential porphyrin and chlorin photosensitizers for PDT. J. Photochem. Photobiol. B Biol. 1996, 33, 171–180. [Google Scholar] [CrossRef]
- Siraj, N.; Kolic, P.E.; Regmi, B.P.; Warner, I.M. Strategy for Tuning the Photophysical Properties of Photosensitizers for Use in Photodynamic Therapy. Chem. Eur. J. 2015, 21, 14440–14446. [Google Scholar] [CrossRef]
- Dąbrowski, J.M.; Pucelik, B.; Regiel-Futyra, A.; Brindell, M.; Mazuryk, O.; Kyzioł, A.; Stochel, G.; Macyk, W.; Arnaut, L.G. Engineering of relevant photodynamic processes through structural modifications of metallotetrapyrrolic photosensitizers. Coord. Chem. Rev. 2016, 325, 67–101. [Google Scholar] [CrossRef]
- Dąbrowski, J.M.; Krzykawska, M.; Arnaut, L.G.; Pereira, M.M.; Monteiro, C.J.P.; Simões, S.; Urbańska, K.; Stochel, G. Tissue Uptake Study and Photodynamic Therapy of Melanoma-Bearing Mice with a Nontoxic, Effective Chlorin. ChemMedChem 2011, 6, 1715–1726. [Google Scholar] [CrossRef]
- Friães, S.; Lima, E.; Boto, R.E.; Ferreira, D.; Fernandes, J.R.; Ferreira, L.F.V.; Silva, A.M.; Reis, L.V. Photophysicochemical Properties and In Vitro Phototherapeutic Effects of Iodoquinoline- and Benzothiazole-Derived Unsymmetrical Squaraine Cyanine Dyes. Appl. Sci. 2019, 9, 5414. [Google Scholar] [CrossRef] [Green Version]
- Jarvi, M.T.; Patterson, M.S.; Wilson, B.C. Insights into Photodynamic Therapy Dosimetry: Simultaneous Singlet Oxygen Luminescence and Photosensitizer Photobleaching Measurements. Biophys. J. 2012, 102, 661–671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baptista, M.S.; Cadet, J.; Di Mascio, P.; Ghogare, A.A.; Greer, A.; Hamblin, M.R.; Lorente, C.; Nunez, S.C.; Ribeiro, M.S.; Thomas, A.H.; et al. Type I and Type II Photosensitized Oxidation Reactions: Guidelines and Mechanistic Pathways. Photochem. Photobiol. 2017, 93, 912–919. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vieira Ferreira, L.F.; Ferreira, D.P.; Duarte, P.; Oliveira, A.; Torres, E.; Ferreira Machado, I.; Almeida, P.; Reis, L.; Santos, P. Surface Photochemistry: 3,3 ’-Dialkylthia and Selenocarbocyanine Dyes Adsorbed onto Microcrystalline Cellulose. Int. J. Mol. Sci. 2012, 13, 596–611. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, I.; Ahmed, S.; Anwar, Z.; Sheraz, M.A.; Sikorski, M. Photostability and Photostabilization of Drugs and Drug Products. Int. J. Photoenergy 2016, 2016, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Welankiwar, A.; Saudagar, S.; Kumar, J.; Barabde, A. Photostability testing of pharmaceutical products. Int. Res. J. Pharm. 2013, 2, 11–15. [Google Scholar] [CrossRef]
- Kawakami, C.M.; Gaspar, L.R. Mangiferin and naringenin affect the photostability and phototoxicity of sunscreens containing avobenzone. J. Photochem. Photobiol. B Biol. 2015, 151, 239–247. [Google Scholar] [CrossRef]
- Menezes, P.F.C.; Imasato, H.; Ferreira, J.; Bagnato, V.S.; Sibata, C.H.; Perussi, J.R. Aggregation susceptibility on phototransformation of hematoporphyrin derivatives. Laser Phys. Lett. 2008, 5, 227–235. [Google Scholar] [CrossRef]
- Tardivo, J.P.; Del Giglio, A.; de Oliveira, C.S.; Gabrielli, D.S.; Junqueira, H.C.; Tada, D.B.; Severino, D.; de Fátima Turchiello, R.; Baptista, M.S. Methylene blue in photodynamic therapy: From basic mechanisms to clinical applications. Photodiagnosis Photodyn. Ther. 2005, 2, 175–191. [Google Scholar] [CrossRef]
- Wagner, M.; Suarez, E.R.; Theodoro, T.R.; Machado Filho, C.D.A.S.; Gama, M.F.M.; Tardivo, J.P.; Paschoal, F.M.; Pinhal, M.A.S. Methylene blue photodynamic therapy in malignant melanoma decreases expression of proliferating cell nuclear antigen and heparanases: MB-PDT decreases PCNA and heparanases. Clin. Exp. Dermatol. 2012, 37, 527–533. [Google Scholar] [CrossRef]
- Hamid, R.; Rotshteyn, Y.; Rabadi, L.; Parikh, R.; Bullock, P. Comparison of alamar blue and MTT assays for high through-put screening. Toxicol. Vitr. 2004, 18, 703–710. [Google Scholar] [CrossRef]
- Burdo, R.H.; Rice-Evans, C. Free Radicals and the Regulation of Mammalian Cell Proliferation. Free Radic. Res. Commun. 1989, 6, 345–358. [Google Scholar] [CrossRef] [PubMed]
- Burdon, R.H.; Gill, V.; Rice-Evans, C. Oxidative Stress and Tumour Cell Proliferation. Free Radic. Res. Commun. 1990, 11, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Day, R.M.; Suzuki, Y.J. Cell Proliferation, Reactive Oxygen and Cellular Glutathione. Dose-Response 2005, 3. [Google Scholar] [CrossRef] [PubMed]
- International Standard (ISO). Biological Evaluation of Medical Devices. Part 5: Tests for In Vitro Cytotoxicity, 3rd ed.; ISO-10993-5; International Standard (ISO): Geneva, Switzerland, 2009. [Google Scholar]
- Terpetschnig, E.; Szmacinski, H.; Lakowicz, J.R. An investigation of squaraines as a new class of fluorophores with long-wavelength excitation and emission. J. Fluoresc. 1993, 3, 153–155. [Google Scholar] [CrossRef] [PubMed]
- Rawla, P.; Sunkara, T.; Barsouk, A. Epidemiology of colorectal cancer: Incidence, mortality, survival, and risk factors. Przegląd Gastroenterol. 2019, 14, 89–103. [Google Scholar] [CrossRef]
- Tunissiolli, N.M.; Castanhole-Nunes, M.M.U.; Biselli-Chicote, P.M.; Pavarino, É.C.; da Silva, R.; da Silva, R.d.C.M.A.; Goloni-Bertollo, E.M. Hepatocellular Carcinoma: A Comprehensive Review of Biomarkers, Clinical Aspects, and Therapy. APJCP 2017, 18. [Google Scholar] [CrossRef]







| Solvent | Dye 1 | Dye 2 | Dye 3 | Dye 4 | Dye 5 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| λmax | log ε | λmax | log ε | λmax | log ε | λmax | log ε | λmax | log ε | |
| Organic Solvent | ||||||||||
| ACN | 668 | 5.05 | 673 | 4.93 | 672 | 5.04 | 683 | 5.07 | 681 | 5.06 |
| ACT | 674 | 5.05 | 680 | 4.94 | 678 | 4.98 | 688 | 5.06 | 687 | 5.03 |
| DCM | 680 | 5.20 | 686 | 5.03 | 685 | 4.96 | 696 | 5.11 | 694 | 5.20 |
| DMF | 676 | 5.04 | 681 | 4.92 | 679 | 4.96 | 689 | 5.04 | 688 | 5.03 |
| DMSO | 681 | 5.27 | 680 | 5.11 | 678 | 5.16 | 688 | 5.20 | 689 | 4.96 |
| DXN | 685 | 5.03 | 691 | 4.97 | 692 | 5.01 | 700 | 5.06 | 699 | 5.08 |
| EtOH | 671 | 5.05 | 674 | 4.92 | 673 | 4.98 | 683 | 5.02 | 682 | 5.03 |
| THF | 685 | 5.05 | 691 | 4.91 | 691 | 4.97 | 700 | 5.07 | 698 | 5.06 |
| Aqueous Solvent | ||||||||||
| DMEM 1 | 693 | 4.38 | 697 | 4.37 | 703 | 4.39 | 710 | 4.19 | 707 | 4.39 |
| H2O 1 | 682 | 4.46 | 688 | 4.43 | 689 | 4.46 | 693 | 4.29 | 696 | 4.49 |
| PBS 1 | 683 | 4.40 | 688 | 4.42 | 686 | 4.44 | 695 | 4.23 | 689 | 4.47 |
| Singlet Oxygen Quantum Yield (ΦΔ) | ||||||||||
| CFM | 0.01 | 0.03 | 0.03 | 0.06 | 0.08 | |||||
| Dye | Irradiation Time (min) | Caco-2 | HepG2 | ||
|---|---|---|---|---|---|
| 1 h | 24 h | 1 h | 24 h | ||
| 1 | 0 | >5 | >5 | >5 | >5 |
| 7 | 0.173 | 0.536 | 0.285 | 0.241 | |
| 14 | 0.563 | 0.244 | 0.259 | 0.263 | |
| 2 | 0 | >5 | >5 | >5 | >5 |
| 7 | >5 | >5 | >5 | >5 | |
| 14 | 2.520 | 1.426 | 1.432 | 1.016 | |
| 3 | 0 | >5 | >5 | >5 | >5 |
| 7 | >5 | 0.983 | 3.661 | 0.768 | |
| 14 | 1.306 | 0.345 | 0.116 | 0.155 | |
| 4 | 0 | >5 | >5 | >5 | >5 |
| 7 | >5 | >5 | >5 | >5 | |
| 14 | >5 | 2.943 | >5 | 4.935 | |
| 5 | 0 | >5 | >5 | >5 | >5 |
| 7 | 0.193 | 0.094 | 0.263 | 0.188 | |
| 14 | 0.079 | 0.032 | 0.143 | 0.108 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
D. Martins, T.; Lima, E.; E. Boto, R.; Ferreira, D.; R. Fernandes, J.; Almeida, P.; F. V. Ferreira, L.; Silva, A.M.; V. Reis, L. Red and Near-Infrared Absorbing Dicyanomethylene Squaraine Cyanine Dyes: Photophysicochemical Properties and Anti-Tumor Photosensitizing Effects. Materials 2020, 13, 2083. https://doi.org/10.3390/ma13092083
D. Martins T, Lima E, E. Boto R, Ferreira D, R. Fernandes J, Almeida P, F. V. Ferreira L, Silva AM, V. Reis L. Red and Near-Infrared Absorbing Dicyanomethylene Squaraine Cyanine Dyes: Photophysicochemical Properties and Anti-Tumor Photosensitizing Effects. Materials. 2020; 13(9):2083. https://doi.org/10.3390/ma13092083
Chicago/Turabian StyleD. Martins, Tiago, Eurico Lima, Renato E. Boto, Diana Ferreira, José R. Fernandes, Paulo Almeida, Luis F. V. Ferreira, Amélia M. Silva, and Lucinda V. Reis. 2020. "Red and Near-Infrared Absorbing Dicyanomethylene Squaraine Cyanine Dyes: Photophysicochemical Properties and Anti-Tumor Photosensitizing Effects" Materials 13, no. 9: 2083. https://doi.org/10.3390/ma13092083