First Electrochemical Sensor (Screen-Printed Carbon Electrode Modified with Carboxyl Functionalized Multiwalled Carbon Nanotubes) for Ultratrace Determination of Diclofenac
Abstract
:1. Introduction
2. Materials and Methods
2.1. Apparatus
2.2. Reagents
2.3. DF Voltammetric Analysis
2.4. DF Chromatographic Analysis
2.5. Real Sample Application
3. Results and Discussion
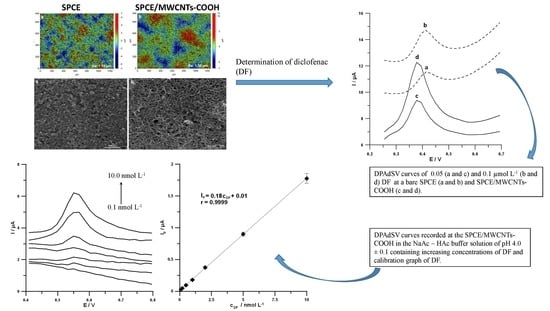
3.1. Characteristics of SPCE/MWCNTs-COOH Sensors
3.2. Optimization of Measurements Solution Composition
3.3. CV Behaviors of DF with the SPCE/MWCNTs-COOH
3.4. Optimization of DPAdSV Parameters
3.5. Analytical Characteristics
3.6. Selectivity of the SPCE/MWCNTs-COOH
3.7. Application in Environmental Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mekassa, B.; Baker, P.G.L.; Chandravanshi, B.S.; Tessema, M. Synthesis, characterization, and preparation of nickel nanoparticles decorated electrochemically reduced graphene oxide modified electrode for electrochemical sensing of diclofenac. J. Solid State Electrochem. 2018, 22, 3607–3619. [Google Scholar] [CrossRef]
- Valcarcel, Y.; Gonzales Alonso, S.; Rodriguez-Gil, J.L.; Romo Maroto, R.; Gil, A.; Catala, M. Analysis of the presence of cardiovascular and analgesic/anti-inflammatory/antipyretic pharmaceutical in rivier- and drinking- water of the Madrid Region in Spain. Chemosphere 2011, 82, 1062–1071. [Google Scholar] [CrossRef] [PubMed]
- Kosjek, T.; Heath, E.; Krbavcic, A. Determination of non-steroidal anti-inflammatory drug (NSAIDs) residues in water samples. Environ. Int. 2005, 31, 679–685. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, L.; Meric, S.; Kassinos, D.; Guida, M.; Russo, F.; Belgiorno, V. Degradation of diclofenac by TiO2 photocatalysis: UV absorbance kinetics and process evaluation through a set of toxicity bioassays. Water Res. 2009, 43, 979–988. [Google Scholar] [CrossRef] [PubMed]
- Taggart, M.A.; Senacha, K.R.; Green, R.E.; Jhala, Y.V.; Raghavan, B.; Rahmani, A.R.; Cuthbert, R.; Pain, D.J.; Meharg, A.E. Diclofenac residues in carcasses of domestic ungulates available to vultures in India. Environ. Int. 2007, 33, 759–765. [Google Scholar] [CrossRef] [PubMed]
- Sanderson, H.; Johnson, D.; Wilson, C.; Brain, R.; Solomon, K. Probabilistic hazard assessment of environmentally occurring pharmaceuticals toxicity to fish, daphnids and algae by ECOSAR screening. Toxicol. Lett. 2003, 144, 383–395. [Google Scholar] [CrossRef]
- Cleuvers, M. Mixture toxicity of the anti-inflammatory drugs diclofenac, ibuprofen, naproxen, and acetylsalicylic acid. Ecotoxicol. Environ. Saf. 2004, 59, 309–315. [Google Scholar] [CrossRef]
- Pandey, G. Spectrophotometric, chromatographic and spectrofluorometric methods for the determination of diclofenac: A review. Pharm. Lett. 2011, 3, 257–265. [Google Scholar]
- Gouda, A.A.; Kotb El-Sayed, M.I.; Amin, A.S.; Sheikh, R.E.L. Spectrophotometric and spectrofluorometric methods for the determination of non-steroidal anti-inflammatory drugs: A review. Arab. J. Chem. 2013, 6, 145–163. [Google Scholar] [CrossRef] [Green Version]
- Heli, H.; Jabbari, A.; Majdi, S.; Mahjoub, M.; Moosavi-Movahedi, A.A.; Sheibani, S.H. Electrooxidation and determination of some non-steroidal anti-inflammatory drugs on nanoparticles of Ni–curcumin-complex-modified electrode. J. Solid State Electrochem. 2009, 13, 1951–1958. [Google Scholar] [CrossRef]
- Afkhami, A.; Bahiraei, A.; Madrakian, T. Gold nanoparticle/multi-walled carbon nanotube modified glassy carbon electrode as a sensitive voltammetric sensor for the determination of diclofenac sodium. Mater. Sci. Eng. C 2016, 59, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Arvand, M.; Gholizadeh, T.M.; Zanjanchi, M.A. MWCNTs/Cu(OH)2 nanoparticles/IL nanocomposite modified glassy carbon electrode as a voltammetric sensor for determination of the non-steroidal anti-inflammatory drug diclofenac. Mater. Sci. Eng. C 2012, 32, 1682–1689. [Google Scholar] [CrossRef] [PubMed]
- Razmi, H.; Sarhang-Zadeh, K.; Mohammad-Rezaei, R. Electrochemical behavior and voltammetric determination of diclofenac at a multi-walled carbon nanotube-ionic liquid composite modified carbon Cceramic electrode. Anal. Lett. 2013, 46, 1885–1896. [Google Scholar] [CrossRef]
- Karuppiah, C.; Cheemalapati, S.; Chen, S.M.; Palanisamy, S. Carboxyl-functionalized graphene oxide-modified electrode for the electrochemical determination of nonsteroidal anti-inflammatory drug diclofenac. Ionics 2015, 21, 231–238. [Google Scholar] [CrossRef]
- Jiokenga, S.L.Z.; Tonlea, I.K.; Walcariusb, A. Amino-attapulgite/mesoporous silica composite films generated by electroassisted self-assembly for the voltammetric determination of diclofenac. Sens. Actuators B Chem. 2019, 287, 296–305. [Google Scholar] [CrossRef]
- Okoth, O.K.; Yan, K.; Liu, L.; Zhang, J. Simultaneous electrochemical determination of paracetamol and diclofenac based on poly(diallyldimethylammonium chloride) functionalized graphene. Electroanalysis 2016, 28, 76–82. [Google Scholar] [CrossRef]
- Yang, X.; Wang, F.; Hu, S. Enhanced oxidation of diclofenac sodium at a nano-structured electrochemical sensing film constructed by multi-wall carbon nanotubes–surfactant composite. Mater. Sci. Eng. C 2008, 28, 188–194. [Google Scholar] [CrossRef]
- Kashefi-Kheyrabadi, L.; Mehrgardi, M.A. Design and construction of a label free aptasensor for electrochemical detection of sodium diclofenac. Biosens. Bioelectron. 2012, 33, 184–189. [Google Scholar] [CrossRef]
- Kamenická, B.; Bartášková, A.; Švancara, I.; Weidlich, T. Applicability of voltammetric determination of diclofenac at carbon paste electrodes to the analysis of aqueous solutions purified by adsorption and/or ionic liquid based ion exchange. Mon. Chem. 2019, 150, 429–437. [Google Scholar] [CrossRef]
- Damiri, S.; Oskoei, Y.M.; Fouladgar, M. Highly sensitive voltammetric and impedimetric sensor based on an ionic liquid/cobalt hexacyanoferrate nanoparticle modified multiwalled carbon nanotubes electrode for diclofenac analysis. J. Exp. Nanosci. 2016, 11, 1384–1401. [Google Scholar] [CrossRef] [Green Version]
- Arvand, M.; Hassannezhad, M. Square wave voltammetric determination of uric acid and diclofenac on multi-walled carbon nanotubes decorated with magnetic core-shell Fe3O4@SiO2 nanoparticles as an enhanced sensing interface. Ionics 2015, 21, 3245–3256. [Google Scholar] [CrossRef]
- Mokhtaria, A.; Karimi-Malehb, H.; Ensafic, A.A.; Beitollahi, H. Application of modified multiwall carbon nanotubes paste electrode for simultaneous voltammetric determination of morphine and diclofenac in biological and pharmaceutical samples. Sens. Actuators B Chem. 2012, 169, 96–105. [Google Scholar] [CrossRef]
- Ensafi, A.A.; Izadi, M.; Karimi-Maleh, H. Sensitive voltammetric determination of diclofenac using room-temperature ionic liquid-modified carbon nanotubes paste electrode. Ionics 2013, 19, 137–144. [Google Scholar] [CrossRef]
- Goodarzian, M.; Khalilzade, M.A.; Karimi, F.; Gupta, V.K.; Keyvanfard, M.; Bagheri, H.; Fouladgar, M. Square wave voltammetric determination of diclofenac in liquid phase using a novel ionic liquid multiwall carbon nanotubes paste electrode. J. Mol. Liq. 2014, 197, 114–119. [Google Scholar] [CrossRef]
- Pourghobadi, R.; Baezzat, M.R. Silica nanoparticles modified carbon paste electrode as a voltammetric sensor for determination of diclofenac. Anal. Bioanal. Chem. Res. 2017, 4, 261–268. [Google Scholar]
- Chethana, B.K.; Basavanna, S.; Naik, Y.A. Voltammetric determination of diclofenac sodium using tyrosine-modified carbon paste electrode. Ind. Eng. Chem. Res. 2012, 51, 10287–10295. [Google Scholar] [CrossRef]
- Blanco-Lopez, M.C.; Fernandez-Llano, L.; Lobo-Castanon, M.J.; Miranda-Ordieres, A.J.; Tunon-Blanco, P. Voltammetry of diclofenac at graphite, carbon Composites, and molecularly imprinted polymer-composite electrodes. Anal. Lett. 2004, 37, 915–927. [Google Scholar] [CrossRef]
- Goyal, R.N.; Chatterjee, S.; Agrawal, B. Electrochemical investigations of diclofenac at edge plane pyrolytic graphite electrode and its determination in human urine. Sens. Actuators B Chem. 2010, 145, 743–748. [Google Scholar] [CrossRef]
- Manea, F.; Ihos, M.; Remes, A.; Burtica, G.; Schoonmanc, J. Electrochemical determination of diclofenac sodium in aqueous solution on Cu-doped zeolite-expanded graphite-epoxy electrode. Electroanalysis 2010, 22, 2058–2063. [Google Scholar] [CrossRef]
- Sarhang-Zadeh, K.; Khatami, A.A.; Jabbari, M.; Bahari, S. Simultaneous determination of diclofenac and indomethacin using a sensitive electrochemical sensor based on multiwalled carbon nanotube and ionic liquid nanocomposite. J. Appl. Electrochem. 2013, 43, 1217–1224. [Google Scholar] [CrossRef]
- Ihosa, M.; Remesb, A.; Maneab, F. Electrochemical determination of diclofenac using boron-doped diamond Electrode. J. Environ. Prot. Ecol. 2012, 13, 2096–2103. [Google Scholar]
- Yilmaz, B.; Kaban, S.; Akcay, B.K.; Ciltas, U. Differential pulse voltammetric determination of diclofenac in pharmaceutical preparations and human serum. Braz. J. Pharm. Sci. 2015, 51, 285–294. [Google Scholar] [CrossRef] [Green Version]
- Ciltas, U.; Yilmaz, B.; Kaban, S.; Akcay, B.K.; Nazik, G. Square wave voltammetric determination of diclofenac in pharmaceutical preparations and human serum. Iran. J. Pharm. Res. 2015, 14, 715–722. [Google Scholar] [PubMed]
- Renedo, O.D.; Alonso-Lomillo, M.A.; Arcos Martinez, M.J. Recent development in the field of screen-printed electrodes and their related applications. Talanta 2007, 73, 202–219. [Google Scholar] [CrossRef] [PubMed]
- Golzari Aqda, T.; Behkami, S.; Bagheri, H. Porous eco–friendly fibers for on–line micro solid–phase extraction of nonsteroidal anti–inflammatory drugs from urine and plasma samples. J. Chromatogr. A 2018, 1574, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Sipa, K.; Brycht, M.; Leniart, A.; Nosal-Wiercińska, A.; Skrzypek, S. Improved electroanalytical characteristics for the determination of pesticide metobromuron in the presence of nanomaterials. Anal. Chim. Acta 2018, 1030, 61–69. [Google Scholar] [CrossRef]
- Cid-Cerón, M.M.; Guzmán-Hernández, D.S.; Ramírez-Silva, M.T.; Galano, A.; Romero-Romo, M.; Palomar-Pardavé, M. New insights on the kinetics and mechanism of the electrochemical oxidation of diclofenac in neutral aqueous medium. Electrochim. Acta 2016, 199, 92–98. [Google Scholar] [CrossRef]
- Tyszczuk-Rotko, K.; Pietrzak, K.; Sasal, A. Adsorptive stripping voltammetric method for the determination of caffeine at integrated three-electrode screen-printed sensor with carbon/carbon nanofibers working electrode. Adsorption 2019, 25, 913–921. [Google Scholar] [CrossRef] [Green Version]
- Laviron, E. General expression of the linear potential sweep voltammogram in the case of diffusionless electrochemical systems. J. Electroanal. Chem. 1979, 101, 19–28. [Google Scholar] [CrossRef]
- Medsen, K.G.; Skonberg, C.; Jurva, U.; Cornett, C.; Hansen, S.H.; Johansen, T.N.; Olsen, J. Bioactivation of diclofenac in vitro and In Vivo: Correlation to electrochemical studies. Chem. Res. Toxicol. 2008, 21, 1107–1119. [Google Scholar] [CrossRef]
- Mocak, J.; Bond, A.M.; Mitchell, S.; Scollary, G. A statistical overview of standard (IUPAC and ACS) and new procedures for determining the limits of detection and quantification: Application to voltammetric and stripping techniques. Pure Appl. Chem. 1997, 69, 297–328. [Google Scholar] [CrossRef]








| Calculated parameter | SPCE | SPCE/MWCNTs-COOH |
|---|---|---|
| ΔE for v of 175 mV s−1 | 189.0 ± 1.9 mV (n = 3) | 149.0 ± 1.5 mV (n = 3) |
| χ0 for v of 175 mV s−1 | 3.26 ± 0.031 (n = 3) | 2.57 ± 0.025 (n = 3) |
| As for v of 5–500 mV s−1 | 0.061 ± 0.00058 cm2 (n = 3) | 0.10 ± 0.00097 cm2 (n = 3) |
| Parameter | DPAdSV |
|---|---|
| Linear range (nmol L−1) | 0.1–10.0 |
| Accumulation time (s) | 60 |
| Slope (b) ± SDb (n = 3) (µA/nmol L−1) | 0.18 ± 0.0070 |
| Intercept (a) ± SDa (n = 3) (µA) | 0.010 ± 0.0017 |
| Correlation coefficient (r) | 0.9999 |
| Limit of detection (LOD; nmol L−1) | 0.028 |
| Limit of quantification (LOQ; nmol L−1) | 0.094 |
| Intra-day precision (RSD, n = 10) (%) | 0.7 |
| Inter-day precision (RSD, n = 15) (%) | 2.1 |
| Reproducibility (RSD, n = 9) (%) | 2.9 |
| Electrode | Method | Linear Range (mol L−1) | Detection Limit (mol L−1) | Application | Ref. |
|---|---|---|---|---|---|
| n-GCE | CV | 2.0 × 10−4–1.5 × 10−3 | 2.8 × 10−5 | pharmaceutical formulations | [10] |
| NiNPs/ERGO/GCE | SWV | 2.5 × 10−7–1.3 × 10−4 | 9.0 × 10−8 | pharmaceutical formulations, urine samples | [1] |
| AuNP/MWCNT/GCE | SWV | 3.0 × 10−8–2.0 × 10−4 | 2.0 × 10−8 | pharmaceutical formulations, urine samples | [11] |
| MWCNTs/ Cu(OH)2/IL/GCE | DPV | 1.8 × 10−7–1.2 × 10−4 | 4.0 × 10−8 | pharmaceutical formulations | [12] |
| MWCNT-IL/CCE | DPV | 5.0 × 10−8–2.0 × 10−5 | 2.7 × 10−8 | blood plasma samples | [13] |
| GO-COOH/GCE | LSV | 1.2 × 10−6–4.0 × 10−4 | 9.0 × 10−8 | urine samples, blood serum samples | [14] |
| GCE/Amino-AT | SWV | 3.0 × 10−7–2.0 × 10−5 | 2.0 × 10−7 | pharmaceutical formulations, spiked water samples | [15] |
| GCE/APTES-Amino-AT-Silica | 5.3 × 10−8 | ||||
| PDDA-GR/GCE | DPV | 1.0 × 10−5–1.0 × 10−4 | 6.1 × 10−7 | pharmaceutical formulations, spiked lake water samples | [16] |
| MWNTs–DHP/GCE | CV | 1.7 × 10−7–2.5 × 10−6 2.5 × 10−6–7.5 × 10−5 | 8.0 × 10−8 | pharmaceutical formulations | [17] |
| DBA/GCE | CV | 1.0 × 10−5–1.0 × 10−3 | 2.7 × 10−7 | blood serum samples | [18] |
| CPE | SWV | 1.0 × 10−6–1.0 × 10−5 | 2.0 × 10−7 | spiked model water samples | [19] |
| MWCNTs/CoHCF/IL/PE | DPV | 1.0 × 10−3–1.0 × 10−1 | 3.0 × 10−4 | pharmaceutical formulations, urine samples | [20] |
| Fe3O4@SiO2/MWCNTs-CPE | SWV | 5.0 × 10−7–1.0 × 10−4 | 4.0 × 10−8 | pharmaceutical formulations, blood serum samples | [21] |
| VFMCNTPE | SWV | 2.5 × 10−6–6.0 × 10−4 | 2.0 × 10−6 | pharmaceutical formulations, urine samples | [22] |
| IL/CNTPE | DPV | 5.0 × 10−7–3.0 × 10−4 | 2.0 × 10−7 | pharmaceutical formulations, urine samples | [23] |
| IL/CNTPE | SWV | 3.0 × 10−7–7.5 × 10−4 | 9.0 × 10−8 | pharmaceutical formulations, urine samples | [24] |
| Silica NPs-CPE | DPV | 1.0 × 10−7–5.0 × 10−4 | 4.6 × 10−8 | pharmaceutical formulations | [25] |
| TCPE | DPV | 1.0 × 10−5–1.4 × 10−4 | 3.3 × 10−6 | pharmaceutical formulations, urine samples | [26] |
| PTFE-G; EG; E-CB | DPV | 6.0 × 10−8–1.0 × 10−6 | 5.0 × 10−8 | pharmaceutical formulations | [27] |
| EPPG | SWV | 1.0 × 10−8–1.0 × 10−6 | 6.2 × 10−9 | pharmaceutical formulations, urine samples | [28] |
| CuZEGE | CV, DPV | 2.0 × 10−5–3.0 × 10−7 | 5.0 × 10−8 | - | [29] |
| MWCNT-IL/CCE | DPV | 5.0 × 10−8–5.0 × 10−5 | 1.8 × 10−8 | pharmaceutical formulations, blood plasma samples | [30] |
| BDDE | DPV | 3.1 × 10−7–3.1 × 10−5 | 3.0 × 10−8 | spiked tap water samples | [31] |
| PtDE | DPV | 5.0 × 10−6–5.9 × 10−5 | 1.0 × 10−6 | pharmaceutical formulations, blood serum samples | [32] |
| PtDE | SWV | 5.1 × 10−6–5.9 × 10−5 | 1.7 × 10−6 | pharmaceutical preparations, blood serum samples | [33] |
| SPCE/MWCNTs-COOH | DPAdSV | 1.0 × 10−10–1.0 × 10−8 | 2.8 × 10−11 | river water samples | This work |
| Sample | DF concentration ± SD (nmol L–1) (n = 3) | Recovery (%) | texp | ||
|---|---|---|---|---|---|
| Added | Found with the DPAdSV procedure | Found with the HPLC/PAD method | DPAdSV | ||
| #1 | 0 | 0.42 ± 0.08 | - | - | - |
| #1 | 5.0 | 5.40 ± 0.20 | - | 99.6 | - |
| #1 | 50.0 | 50.80 ± 1.40 | 52.30 ± 4.08 | 100.5 | 0.60 |
| #2 | 0 | - | - | - | - |
| #2 | 0.4 | 0.40 ± 0.01 | - | 100.0 | - |
| #2 | 5.0 | 5.38 ± 0.33 | - | 99.6 | - |
| #2 | 50.0 | 51.0 ± 0.90 | 49.80 ± 4.25 | 100.9 | 0.48 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sasal, A.; Tyszczuk-Rotko, K.; Wójciak, M.; Sowa, I. First Electrochemical Sensor (Screen-Printed Carbon Electrode Modified with Carboxyl Functionalized Multiwalled Carbon Nanotubes) for Ultratrace Determination of Diclofenac. Materials 2020, 13, 781. https://doi.org/10.3390/ma13030781
Sasal A, Tyszczuk-Rotko K, Wójciak M, Sowa I. First Electrochemical Sensor (Screen-Printed Carbon Electrode Modified with Carboxyl Functionalized Multiwalled Carbon Nanotubes) for Ultratrace Determination of Diclofenac. Materials. 2020; 13(3):781. https://doi.org/10.3390/ma13030781
Chicago/Turabian StyleSasal, Agnieszka, Katarzyna Tyszczuk-Rotko, Magdalena Wójciak, and Ireneusz Sowa. 2020. "First Electrochemical Sensor (Screen-Printed Carbon Electrode Modified with Carboxyl Functionalized Multiwalled Carbon Nanotubes) for Ultratrace Determination of Diclofenac" Materials 13, no. 3: 781. https://doi.org/10.3390/ma13030781