Adaptive Volume Control in Titanium Alloy for High Temperature Performance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Procedure
2.2. First Principles Calculations
3. Results
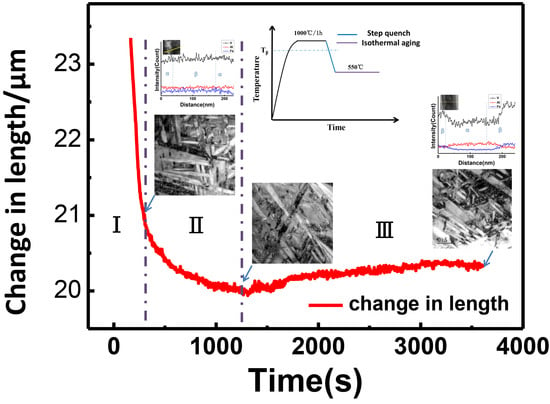
3.1. Dilatometer Measurements
3.2. Change in Structure
3.3. Change in the Composition
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sigmund, O.; Torquato, S. Design of materials with extreme thermal expansion using a three-phase topology optimization method. J. Mech. Phys. Solids 1997, 45, 1037–1067. [Google Scholar] [CrossRef]
- Friedrich, C.; Hubbertz, H. Friction behavior and preload relaxation of fastening systems with composite structures. Compos. Struct. 2014, 110, 335–341. [Google Scholar] [CrossRef]
- Pai, N.G.; Hess, D.P. Three-dimensional finite element analysis of threaded fastener loosening due to dynamic shear load. Eng. Fail. Anal. 2002, 9, 383–402. [Google Scholar] [CrossRef]
- Sun, F.; Laillé, D.; Gloriant, T. Thermal analysis of the ω nanophase transformation from the metastable β Ti–12Mo alloy. J. Therm. Anal. 2010, 101, 81–88. [Google Scholar] [CrossRef]
- Chen, J.; Xiao, W.; Dargusch, M.S.; Ma, C. The Dependence of Isothermal ω Precipitation on the Quenching Rate in a Metastable β-Ti Alloy. Sci. Rep. 2015, 5, 14632. [Google Scholar] [CrossRef]
- Tang, B.; Kou, H.C.; Wang, Y.H.; Zhu, Z.S.; Zhang, F.S.; Li, J.S. Kinetics of orthorhombic martensite decomposition in TC21 alloy under isothermal conditions. J. Mater. Sci. 2012, 47, 521–529. [Google Scholar] [CrossRef]
- Chang, Y.H.; Yang, H.H. Cabin safety and emergency evacuation: Passenger experience of flight CI-120 accident. Accid. Anal. Prev. 2011, 43, 1049–1055. [Google Scholar] [CrossRef]
- Ferrero, J.G. Candidate materials for high-strength fastener applications in both the aerospace and automotive industries. J. Mater. Eng. Perform. 2005, 14, 691–696. [Google Scholar] [CrossRef]
- Bania, P.J. Beta titanium alloys and their role in the titanium industry. In Beta Titanium Alloys in the 1990’s; TSM: Pittsburgh, PA, USA, 1998; pp. 3–14. [Google Scholar]
- Terlinde, G.; Fischer, G. Titanium 95. In Proceedings of the Eighth World Conference on Titanium, Birmingham, UK, 22–26 October 1995; Blenkinsop, P.A., Evans, W.J., Flower, H.M., Eds.; Institute of Materials: London, UK, 1996; Volume III. [Google Scholar]
- Fanning, J.C. Properties of TIMETAL 555 (Ti-5Al-5Mo-5V-3Cr-0.6 Fe). J. Mater. Eng. Perform. 2005, 14, 788–791. [Google Scholar] [CrossRef]
- Mehdi, M.; Farokhzadeh, K.; Edrisy, A. Dry sliding wear behavior of superelastic Ti–10V–2Fe–3Al β-titanium alloy. Wear 2016, 350, 10–20. [Google Scholar] [CrossRef]
- Van Bohemen, S.M.C.; Sietsma, J.; Der Zwaag, S.V. Experimental observations elucidating the mechanisms of structural bcc-hcp transformations in β−Ti alloys. Phys. Rev. B 2006, 74, 134114. [Google Scholar] [CrossRef]
- Nag, S.; Zheng, Y.; Williams, R.E.A.; Devaraj, A.; Boyne, A.; Wang, Y.; Collins, P.C.; Viswanathan, G.B.; Tiley, J.S.; Muddle, B.C.; et al. Non-classical homogeneous precipitation mediated by compositional fluctuations in titanium alloys. Acta Mater. 2012, 60, 6247–6256. [Google Scholar] [CrossRef]
- Boyne, A.; Wang, D.; Shi, R.P.; Zheng, Y.; Behera, A.; Nag, S.; Tiley, J.S.; Fraser, H.L.; Banerjee, R.; Wang, Y. Pseudospinodal mechanism for fine α/β, microstructures in β-Ti alloys. Acta Mater. 2014, 64, 188–197. [Google Scholar] [CrossRef]
- Li, P.; Zhang, T.L.; Sun, X.; Zhang, H.L.; Wang, D.; Sun, Q.Y.; Xiao, L.; Sun, J. Secondary hardening behavior in Ti alloy. Mater. Sci. Eng. A 2019, 759, 640–647. [Google Scholar] [CrossRef]
- Lütjering, G.; Williams, J.C. Titanium, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2007. [Google Scholar]
- Hohenberg, P.; Kohn, W. Inhomogeneous electron gas. Phys. Rev. 1964, 136, 864–871. [Google Scholar] [CrossRef]
- Vitos, L.; Skriver, H.L.; Johansson, B.; Kollár, J. Application of the exact muffin-tin orbitals theory: The spherical cell approximation. Comput. Mater. Sci. 2000, 18, 24–38. [Google Scholar] [CrossRef]
- Malinov, S.; Sha, W.; Markovsky, P. Experimental study and computer modelling of the β⇒α+β phase transformation in β21s alloy at isothermal conditions. J. Alloy. Compd. 2003, 348, 110–118. [Google Scholar] [CrossRef]
- Bein, S.; Bechet, J. Phase Transformation Kinetics and Mechanisms in Titanium Alloys Ti-6.2. 4.6, ß-CEZ and Ti-10.2.3. J. Phys. IV 1996, 6, 1–99. [Google Scholar]
- Lu, X.G.; Selleby, M.; Sundman, B. Assessments of molar volume and thermal expansion for selected bcc, fcc and hcp metallic elements. Calphad-Comput. Coupling Ph. Diagr. Thermochem. 2005, 29, 68–89. [Google Scholar] [CrossRef]
- Aurelio, G.; Guillermet, A.F.; Cuello, G.J.; Campo, J. Metastable phases in the Ti-V system: Part I. Neutron diffraction study and assessment of structural properties. Metall. Mater. Trans. A 2002, 33, 1307–1317. [Google Scholar] [CrossRef]
- Eppelsheimer, D.S.; Penman, R.R. Accurate Determination of the Lattice of Beta-Titanium at 900 °C. Nature 1950, 166, 960. [Google Scholar] [CrossRef]
- Schmitz-Pranghe, N.; Dünner, P. Gitterstruktur und thermische Ausdehnung der Uebergangsmetalle Scandium, Titan, Vanadin und Mangan. Z. Metallkd. 1968, 59, 377–382. [Google Scholar]
- Senkov, O.N.; Chakoumakos, B.C.; Jonas, J.J.; Froes, F.H. Effect of temperature and hydrogen concentration on the lattice parameter of beta titanium. Mater. Res. Bull. 2001, 36, 1431–1440. [Google Scholar] [CrossRef]
- Spreadborough, J.; Christian, J.W. The measurement of the lattice expansions and Debye temperatures of titanium and silver by X-ray method. Proc. Phys. Soc. 1959, 74, 609. [Google Scholar] [CrossRef]
- Margolin, H.; Portisch, H. Hydrogen-Induced Expansions in Titanium-Aluminum Alloys. Trans. Metall. Soc. AIME 1968, 242, 1901. [Google Scholar]
- Pawar, R.R.; Deshpande, V.T. The anisotropy of the thermal expansion of α-titanium. Acta Crystallogr. Sect. A 1968, 24, 316–317. [Google Scholar] [CrossRef]
- Roberts, W.T. Preferred orientation and anisotropy in titanium. J. Less Common Met. 1962, 4, 345–361. [Google Scholar] [CrossRef]
- Willens, R.H. Vacuum X-ray Diffractometer for High Temperature Studies of Metals Sensitive to Contamination by Oxygen and Nitrogen. Rev. Sci. Instrum. 1962, 33, 1069–1076. [Google Scholar] [CrossRef]
- Vegard, L. Die konstitution der mischkristalle und die raumfüllung der atome. Z. Phys. 1921, 5, 17–26. [Google Scholar] [CrossRef]
- Ni, Y.; Khachaturyan, A.G. From chessboard tweed to chessboard nanowire structure during pseudospinodal decomposition. Nat. Mater. 2009, 8, 410. [Google Scholar] [CrossRef]




© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, P.; Sun, X.; Zhang, T.; Zhang, H.; Wang, D.; Sun, Q.; Xiao, L.; Sun, J. Adaptive Volume Control in Titanium Alloy for High Temperature Performance. Materials 2019, 12, 3950. https://doi.org/10.3390/ma12233950
Li P, Sun X, Zhang T, Zhang H, Wang D, Sun Q, Xiao L, Sun J. Adaptive Volume Control in Titanium Alloy for High Temperature Performance. Materials. 2019; 12(23):3950. https://doi.org/10.3390/ma12233950
Chicago/Turabian StyleLi, Pei, Xun Sun, Tianlong Zhang, Hualei Zhang, Dong Wang, Qiaoyan Sun, Lin Xiao, and Jun Sun. 2019. "Adaptive Volume Control in Titanium Alloy for High Temperature Performance" Materials 12, no. 23: 3950. https://doi.org/10.3390/ma12233950