Isothermal Diffusion Behavior and Surface Performance of Cu/Ni Coating on TC4 Alloy
Abstract
:1. Introduction
2. Experiment
2.1. Experimental Material
2.2. Microstructural Observation
2.3. Tribological and Anti-Corrosion Properties
3. Results and Discussion
3.1. The Phase Transitions and Microstructure of the Coating
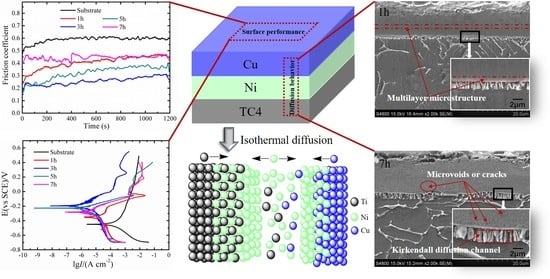
3.2. Effect of Isothermal Treatment on Diffusion Behavior
3.3. Effect of Isothermal Diffusion Behavior on Tribological and Anti-corrosion Properties
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wang, F.; Wang, D. Study on the Effects of Heat-treatment on Mechanical Properties of TC4 Titanium Alloy Sheets for Aviation Application. Titan. Ind. Progress. 2017, 45, 56–60. [Google Scholar]
- Campo, K.N.; Lima, D.D.D.; Lopes, E.S.N.; Caram, R. Erratum to: On the selection of Ti–Cu alloys for thixoforming processes: Phase diagram and microstructural evaluation. J. Mater. Sci. 2016, 51, 9912–9913. [Google Scholar] [CrossRef]
- Chen, S.; Duan, Y.H.; Huang, B.; Hu, W.C. Structural properties, phase stability, elastic properties and electronic structures of Cu–Ti intermetallics. Philos. Mag. 2015, 95, 3535–3553. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, H.L.; Wu, J.H.; Wang, X.T. Enhanced thermal conductivity in copper matrix composites reinforced with titanium-coated diamond particles. Scr. Mater. 2011, 65, 1097–1100. [Google Scholar] [CrossRef]
- Jiang, P.; He, X.L.; Li, X.X.; Wang, H.M. Wear resistance of a laser surface alloyed Ti–6Al–4V alloy. Surf. Coat. Tech. 2000, 130, 24–28. [Google Scholar] [CrossRef]
- Xue, W.B.; Chao, W.; Deng, Z.W.; Li, Z. Ceramic Coatings on TC4 Titanium Alloy Deposited by AC Microarc Oxidation. J. Inorg. Mater. 2001, 17, 326–331. [Google Scholar]
- Yi, X.H.; Fan, Z.G.; Zhang, J.L.; Li, F.H.; Tian, A. Experimental study of preparation of TiO2 porous films on the surface of TC4 titanium alloy by anodic oxidation. J. Mater. Eng. 2010, 24, 38–41. [Google Scholar]
- Shen, Z.C.; Xie, F.Q.; Wu, X.Q.; Yao, X.F. Properties of Coating on TC4 Titanium Alloy by Copper Electroplating. China Surf. Eng. 2012, 25, 45–49. [Google Scholar]
- Yao, X.; Xie, F.; Wang, Y.; Wu, X. Research on Tribological and Wear Properties of Cu Coating on TC4 Alloy. Rare Metal. Mat. Eng. 2012, 41, 2135–2138. [Google Scholar]
- Aydın, K.; Kaya, Y.; Kahraman, N. Experimental study of diffusion welding/bonding of titanium to copper. Mater. Des. 2012, 37, 356–368. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, S.; Zhao, Y.; Liu, Q.; Zhu, L.; Song, X.; Zhang, Y.; Hao, J. Diffusion behavior and mechanical properties of Cu/Ni coating on TC4 alloy. Vacuum 2017, 143, 150–157. [Google Scholar] [CrossRef]
- Tavoosi, M. The Kirkendall Void Formation in Al/Ti Interface during Solid-State Reactive Diffusion between Al and Ti. Surf. Interfaces 2017, 9, 196–200. [Google Scholar] [CrossRef]
- Chen, H.; Zheng, L.J.; Zhang, F.X.; Zhang, H.X. Thermal stability and hardening behavior in superelastic Ni-rich Nitinol alloys with Al addition. Mater. Sci. Eng. A 2017, 708, 514–522. [Google Scholar] [CrossRef]
- Wang, Z.; He, Z.; Wang, Y.; Liu, X.; Tang, B. Microstructure and tribological behaviors of Ti6Al4V alloy treated by plasma Ni alloying. Appl. Surf. Sci. 2011, 257, 10267–10272. [Google Scholar] [CrossRef]
- Wei, H.; Wei, Y.H.; Hou, L.F.; Dang, N. Correlation of ageing precipitates with the corrosion behaviour of Cu-4 wt.% Ti alloys in 3.5 wt.% NaCl solution. Corros. Sci. 2016, 111, 382–390. [Google Scholar] [CrossRef]
- Qin, Z.; Luo, Q.; Wu, Z.; Shen, B.; Liu, L.; Hu, W. The corrosion behavior of Ni-Cu gradient layer on the nickel aluminum-bronze (NAB) alloy. Corros. Sci. 2018, 138, 8–19. [Google Scholar]
- Dehgahi, S.; Amini, R.; Alizadeh, M. Microstructure and corrosion resistance of Ni-Al2O3-SiC nanocomposite coatings produced by electrodeposition technique. J. Alloy. Compd. 2017, 692, 622–628. [Google Scholar] [CrossRef]
- Dai, X.; Cao, J.; Tian, Y.; Chen, Z.; Song, X.; Feng, J. Effect of holding time on microstructure and mechanical properties of SiC/SiC joints brazed by Ag-Cu-Ti + B4C composite filler. Mater. Charact. 2016, 118, 294–301. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, Q.; Sun, D.L.; Han, X.L. Co-effect of heat and direct current on growth of intermetallic layers at the interface of Ti–Ni diffusion couples. J. Alloy. Compd. 2011, 509, 1201–1205. [Google Scholar] [CrossRef]
- Seitz, F. On the porosity observed in the Kirkendall effect. Acta Metall. 1953, 1, 355–369. [Google Scholar] [CrossRef]
- Bastin, G.F.; Rieck, G.D. Diffusion in the titanium-nickel system: I. occurrence and growth of the various intermetallic compounds. Metall. Trans. 1974, 5, 1817–1826. [Google Scholar] [CrossRef]
- Puente, A.E.P.Y.; Dunand, D.C. Synthesis of NiTi microtubes via the Kirkendall effect during interdiffusion of Ti-coated Ni wires. Intermetallics 2018, 92, 42–48. [Google Scholar] [CrossRef]
- Fan, H.J.; Gosele, U.; Zacharias, M. Formation of nanotubes and hollow nanoparticles based on Kirkendall and diffusion processes: A review. Small 2007, 3, 1660–1671. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.B.; Lu, K.; Wilde, G.; Divinski, S.V. Interfacial diffusion in Cu with a gradient nanostructured surface layer. Acta Mater. 2010, 58, 2376–2386. [Google Scholar] [CrossRef]
- Shu, B.P.; Liu, L.; Deng, Y.D.; Zhong, C.; Wu, Y.T.; Shen, B.; Hu, W.B. An investigation of grain boundary diffusion and segregation of Ni in Cu in an electrodeposited Cu/Ni micro-multilayer system. Mater. Lett. 2012, 89, 223–225. [Google Scholar] [CrossRef]
- Abdul-Lettif, A.M. Investigation of interdiffusion in copper-nickel bilayer thin films. Phys. B 2007, 388, 107–111. [Google Scholar] [CrossRef]
- Kundu, S.; Chatterjee, S. Characterization of diffusion bonded joint between titanium and 304 stainless steel using a Ni interlayer. Mater. Charact. 2008, 59, 631–637. [Google Scholar] [CrossRef]
- Yang, C.; Mo, D.G.; Lu, H.Z.; Li, X.Q.; Zhang, W.W.; Fu, Z.Q.; Zhang, L.C.; Lavernia, E. Reaction diffusion rate coefficient derivation by isothermal heat treatment in spark plasma sintering system. Scr. Mater. 2017, 134, 91–94. [Google Scholar] [CrossRef]
- Askeland, D.R.; Phule, P. Essentials of Materials Science and Engineering; Tsinghua University Press: Beijing, China, 2005. [Google Scholar]
- Chen, G.Q.; Ren, X.; Zhou, W.L.; Zhang, J.S. Atomic interdiffusion in Ni–Cu system under high magnetic field. Metal. Soc. 2013, 23, 2460–2464. [Google Scholar] [CrossRef]
- Liu, D.; Huang, D.; Liu, S.; Du, Y.; Divinski, S.V. Composition-dependent tracer diffusion coefficients in the B2Ni-Al-Ti alloy via a combination of radiotracer and diffusion couple techniques. J. Alloy. Compd. 2017, 720, 332–339. [Google Scholar] [CrossRef]
- Simoes, S.; Viana, F.; Ramos, A.S.; Vieira, M.T.; Vieira, M.F. Reaction zone formed during diffusion bonding of TiNi to Ti6Al4V using Ni/Ti nanolayers. J. Mater. Sci. 2013, 48, 7718–7727. [Google Scholar] [CrossRef]
- Shen, Q.; Xiang, H.; Luo, G.; Wang, C.; Li, M.; Zhang, L. Microstructure and mechanical properties of TC4/oxygen-free copper joint with silver interlayer prepared by diffusion bonding. Mater. Sci. Eng. A 2014, 596, 45–51. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, Y.; Zhang, T.; Zhang, W.; Zheludkevich, M.L. Interaction effect between different constituents in silicate-containing electrolyte on PEO coatings on Mg alloy. Surf. Coat. Tech. 2016, 307, 825–836. [Google Scholar] [CrossRef]







| Elements | V | Al | Fe | Mn | Zn | Si | Ti |
|---|---|---|---|---|---|---|---|
| Nominal | 3.3–4.5 | 5.0–6.5 | 0.3–0.9 | 0.5 | 0.3 | 0.4 | Bal |
| Diffusion Time (h) | Ecorr (mV.SCE) | Icorr (mA/cm2) | ba (mV/dec) | bc (mV/dec) |
|---|---|---|---|---|
| Substrate | −455.42 | 11.17 × 10−3 | 132.48 | −63.72 |
| 1 | −354.07 | 6.04 × 10−3 | 99.19 | −125.39 |
| 3 | −201.14 | 0.514 × 10−3 | 96.98 | −59.43 |
| 5 | −231.72 | 0.705 × 10−3 | 52.90 | −73.90 |
| 7 | −276.62 | 2.68 × 10−3 | 101.51 | −121.39 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, N.; Chen, Y.-N.; Zhang, L.; Li, Y.; Liu, S.-S.; Zhan, H.-F.; Zhu, L.-X.; Zhu, S.-D.; Zhao, Y.-Q. Isothermal Diffusion Behavior and Surface Performance of Cu/Ni Coating on TC4 Alloy. Materials 2019, 12, 3884. https://doi.org/10.3390/ma12233884
Wang N, Chen Y-N, Zhang L, Li Y, Liu S-S, Zhan H-F, Zhu L-X, Zhu S-D, Zhao Y-Q. Isothermal Diffusion Behavior and Surface Performance of Cu/Ni Coating on TC4 Alloy. Materials. 2019; 12(23):3884. https://doi.org/10.3390/ma12233884
Chicago/Turabian StyleWang, Nan, Yong-Nan Chen, Long Zhang, Yao Li, Shuang-Shuang Liu, Hai-Fei Zhan, Li-Xia Zhu, Shi-Dong Zhu, and Yong-Qing Zhao. 2019. "Isothermal Diffusion Behavior and Surface Performance of Cu/Ni Coating on TC4 Alloy" Materials 12, no. 23: 3884. https://doi.org/10.3390/ma12233884